learning objectives
- name alkanes, cycloalkanes, alkenes, alkynes, alkyl halides, ethers, alcohols, amines, benzene and its derivatives, aldehydes, ketones, amines, carboxylic acids, and carboxylic acid derivatives using IUPAC (systematic) and selected common name nomenclature
- draw the structure of alkanes, cycloalkanes, alkenes, alkynes, alkyl halides, ethers, alcohols, amines, benzene and its derivatives, aldehydes, ketones, amines, carboxylic acids, and carboxylic acid derivatives from the IUPAC (systematic) and selected common names
Overview of the IUPAC System for Naming Organic Compounds
The International Union of Pure and Applied Chemistry (IUPAC) has established the rules of nomenclature of all chemical compounds. IUPAC nomenclature can also be called "systematic" nomenclature because there is an overall system and structure to the names. This section provides an overview of the general naming strategy and structure for organic compounds.
Naming organic compounds according to the IU{AC system requires up to four pieces of information
1. recognize & prioritize the functional group(s) present
2. identify & number the longest continuous carbon chain to give the highest ranking group the lowest possible number
3. cite the substituents (branches) alphabetically using the numbering determined above
4. recognize & classify any stereochemistry (E/Z, R/S, cis/trans, etc)
With these four pieces of information, the IUPAC name is written using the format below. This same format applies to ALL the organic compounds.
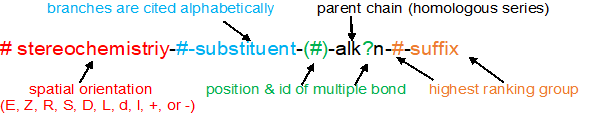
Recognize & Prioritize the Functional Group(s) Present
The IUPAC Rules of Organic Nomenclature assume that the following table is understood and memorized.
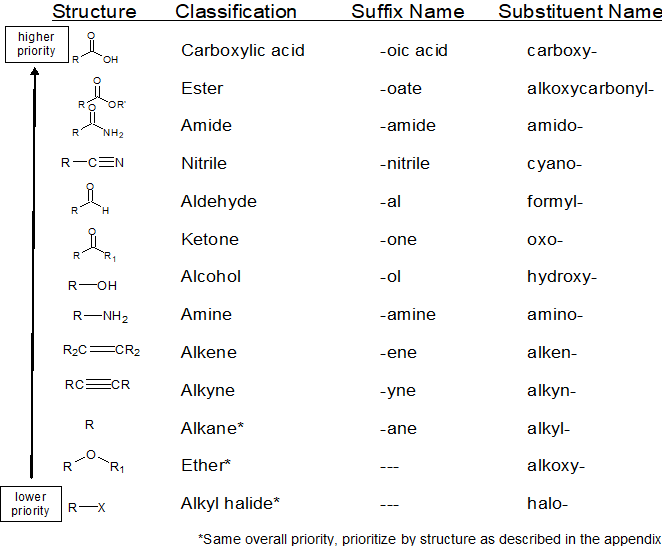
Identify & Number the Longest Continuous Carbon Chain with the Highest Priority Group
The longest continuous carbon chain (parent) is named using the Homologous Series, as well as any carbon branches. The suffixes and location within the name distinguish between the parent and the branches.
When Alkenes and Alkynes have Lower Priority
The hydrocarbon suffixes differ in the letter preceeding the "n". When alkenes and alkynes occur in compounds with higher priority functional groups, then the distinction between hydrocarbons is communicated with a single letter: "e", or "y" for alkenes and alkynes, respectively, An example is shown below to illustrate the application of this rule.

substituents (branches) alphabetically
The major substituents are listed above and need to be memorized. There are a few additional substituents that will introduced later in the text.
Stereochemistry
Distinguishing spatial orientations of atoms (stereochemistry) are communicated at the beginning of the name using the appropriate symbols, such as E/Z, R/S, cis/trans, etc. This nomenclature will be discussed when it is possible for a functional group.




