Radicals
In chemistry, a radical (more precisely, a free radical) is an atom, molecule, or ion that has unpaired valence electrons or an open electron shell, and therefore may be seen as having one or more "dangling" covalent bonds.
With some exceptions, these "dangling" bonds make free radicals highly chemically reactive towards other substances, or even towards themselves: their molecules will often spontaneously dimerize or polymerize if they come in contact with each other. Most radicals are reasonably stable only at very low concentrations in inert media or in a vacuum.
A notable example of a free radical is the hydroxyl radical (HO•), a molecule that is one hydrogen atom short of a water molecule and thus has one bond "dangling" from the oxygen. Two other examples are the carbene molecule (:CH2), which has two dangling bonds; and the superoxide anion (•O−2), the oxygen molecule O2 with one extra electron, which has one dangling bond. In contrast, the hydroxyl anion (HO−), the oxide anion (O2−) and thecarbenium cation (CH+3) are not radicals, since the bonds that may appear to be dangling are in fact resolved by the addition or removal of electrons.
Free radicals may be created in a number of ways, including synthesis with very dilute or rarefied reagents, reactions at very low temperatures, or breakup of larger molecules. The latter can be affected by any process that puts enough energy into the parent molecule, such as ionizing radiation, heat, electrical discharges, electrolysis, and chemical reactions. Indeed, radicals are intermediate stages in many chemical reactions.
Free radicals play an important role in combustion, atmospheric chemistry, polymerization, plasma chemistry, biochemistry, and many other chemical processes. In living organisms, the free radicals superoxide and nitric oxideand their reaction products regulate many processes, such as control of vascular tone and thus blood pressure. They also play a key role in the intermediary metabolism of various biological compounds. Such radicals can even be messengers in a process dubbed redox signaling. A radical may be trapped within a solvent cage or be otherwise bound.
Until late in the 20th century the word "radical" was used in chemistry to indicate any connected group of atoms, such as a methyl group or a carboxyl, whether it was part of a larger molecule or a molecule on its own. The qualifier "free" was then needed to specify the unbound case. Following recent nomenclature revisions, a part of a larger molecule is now called a functional group or substituent, and "radical" now implies "free". However, the old nomenclature may still occur in the literature.
The formation of radicals may involve breaking of covalent bonds homolytically, a process that requires significant amounts of energy. For example, splitting H2 into 2H· has a ΔH° of +435 kJ/mol, and Cl2 into 2Cl· has a ΔH° of +243 kJ/mol. This is known as the homolytic bond dissociation energy, and is usually abbreviated as the symbol ΔH°. The bond energy between two covalently bonded atoms is affected by the structure of the molecule as a whole, not just the identity of the two atoms. Likewise, radicals requiring more energy to form are less stable than those requiring less energy. Homolytic bond cleavage most often happens between two atoms of similar electronegativity. In organic chemistry this is often the O-O bond in peroxide species or O-N bonds. Sometimes radical formation is spin-forbidden, presenting an additional barrier. However, propagation is a very exothermic reaction. Likewise, although radical ions do exist, most species are electrically neutral. Radicals may also be formed by single electron oxidation or reduction of an atom or molecule. An example is the production of superoxide by the electron transport chain. Early studies of organometallic chemistry, especially tetra-alkyl lead species by F.A. Paneth and K. Hahnfeld in the 1930s supported heterolytic fission of bonds and a radical based mechanism.
Depiction in chemical reactions
In chemical equations, free radicals are frequently denoted by a dot placed immediately to the right of the atomic symbol or molecular formula as follows:
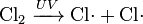
- Chlorine gas can be broken down by ultraviolet light to form atomic chlorine radicals.
Radical reaction mechanisms use single-headed arrows to depict the movement of single electrons:
The homolytic cleavage of the breaking bond is drawn with a 'fish-hook' arrow to distinguish from the usual movement of two electrons depicted by a standard curly arrow. It should be noted that the second electron of the breaking bond also moves to pair up with the attacking radical electron; this is not explicitly indicated in this case.
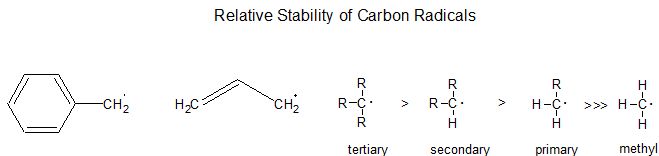
Relative Stability
Radical alkyl intermediates are stabilized by similar physical processes to carbocations: as a general rule, the more substituted the radical center is, the more stable it is. This directs their reactions. Thus, formation of a tertiary radical (R3C·) is favored over secondary (R2HC·), which is favored over primary (RH2C·). Likewise, radicals next to functional groups such as carbonyl, nitrile, and ether are more stable than tertiary alkyl radicals.
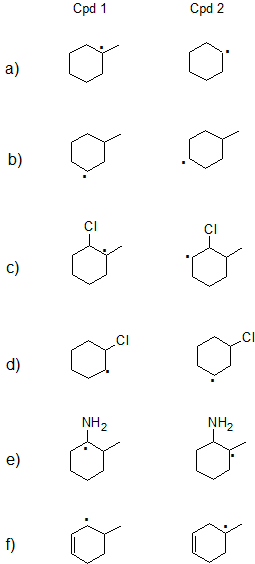
Exercise
1. State which carbon radical (free radical) in each pair below is more stable or if they are expected to have comparable stability. Explain your reasoning.
- Answer
- 1.
a) Cpd 1: Tertiary radicals are more stable than secondary radicals.
b) Cpds 1 and 2 are both secondary so they have comparable stability.
c) Cpd 1: Tertiary radicals are more stable than secondary radicals with similar effects from the Cl atom.
d) Cpd 2: Both compounds are secondary, but positive charge is further from electron-withdrawing chlorine on Cpd 2.
e) Cpd 1: Lone pair on nitrogen can donate electrons by resonance.
f) Cpd 1: Secondary allylic radicals are more stable than tertiary radicals. (Primary allylic radicals are comparable in stability to tertiary radicals.)
-
-