8.3: Electrochemistry- Cells and Batteries
- Last updated
- Save as PDF
- Page ID
- 351262
Learning Objectives
- Define electrochemistry.
- Describe the basic components of electrochemical cells.
- List some of the characteristics, applications and limitations of cells and batteries.
- Know the difference between galvanic and electrolytic cells.
- Define electrolysis and list several of its applications.
Metal exposed to the outside elements will usually corrode if not protected. The corrosion process is a series of redox reactions involving the metal of the sculpture. In some situations, the metals are deliberately left unprotected so that the surface will undergo changes that may enhance the esthetic value of the work.
Electrochemical Reactions
Chemical reactions either absorb or release energy, which can be in the form of electricity. Electrochemistry is a branch of chemistry that deals with the interconversion of chemical energy and electrical energy. Electrochemistry has many common applications in everyday life. All sorts of batteries, from those used to power a flashlight to a calculator to an automobile, rely on chemical reactions to generate electricity. Electricity is used to plate objects with decorative metals like gold or chromium. Electrochemistry is important in the transmission of nerve impulses in biological systems. Redox chemistry, the transfer of electrons, is behind all electrochemical processes.
An electrochemical cell is any device that converts chemical energy into electrical energy or electrical energy into chemical energy. There are three components that make up an electrochemical reaction. There must be a solution where redox reactions can occur. These reactions generally take place in water to facilitate electron and ion movement. A conductor must exist for electrons to be transferred. This conductor is usually some kind of wire so that electrons can move from one site to another. Ions also must be able to move through some form of salt bridge that facilitates ion migration.
Luigi Galvani
Luigi Galvani (1737 - 1798) was an Italian physician and scientist who did research on nerve conduction in animals. His accidental observation of the twitching of frog legs when they were in contact with an iron scalpel while the legs hung on copper hooks led to studies on electrical conductivity in muscles and nerves. He believed that animal tissues contained an "animal electricity" similar to the natural electricity that caused lightning to form.

Alessandro Volta developed the first "voltaic cell" in 1800. This battery consisted of alternating disks of zinc and silver with pieces of cardboard soaked in brine separating the disks. Since there were no voltmeters at the time (and no idea that the electric current was due to electron flow), Volta had to rely on another measure of battery strength: the amount of shock produced (it's never a good idea to test things on yourself). He found that the intensity of the shock increased with the number of metal plates in the system. Devices with twenty plates produced a shock that was quite painful. It's a good thing we have voltmeters today to measure electric current instead of the "stick your finger on this and tell me what you feel" method.
Galvanic (Voltaic) Cells
Galvanic cells, also known as voltaic cells, are electrochemical cells in which spontaneous oxidation-reduction reactions produce electrical energy. In writing the equations, it is often convenient to separate the oxidation-reduction reactions into half-reactions to facilitate balancing the overall equation and to emphasize the actual chemical transformations.
Consider what happens when a clean piece of copper metal is placed in a solution of silver nitrate (Figure \(\PageIndex{1}\)). As soon as the copper metal is added, silver metal begins to form and copper ions pass into the solution. The blue color of the solution on the far right indicates the presence of copper ions. The reaction may be split into its two half-reactions. Half-reactions separate the oxidation from the reduction, so each can be considered individually.
&\textrm{oxidation: }\ce{Cu}(s)⟶\ce{Cu^2+}(aq)+\ce{2e-}\\
&\underline{\textrm{reduction: }2×(\ce{Ag+}(aq)+\ce{e-}⟶\ce{Ag}(s))\hspace{40px}\ce{or}\hspace{40px}\ce{2Ag+}(aq)+\ce{2e-}⟶\ce{2Ag}(s)}\\
&\textrm{overall: }\ce{2Ag+}(aq)+\ce{Cu}(s)⟶\ce{2Ag}(s)+\ce{Cu^2+}(aq)
\end{align*} \nonumber \]
The equation for the reduction half-reaction had to be doubled so the number electrons “gained” in the reduction half-reaction equaled the number of electrons “lost” in the oxidation half-reaction.
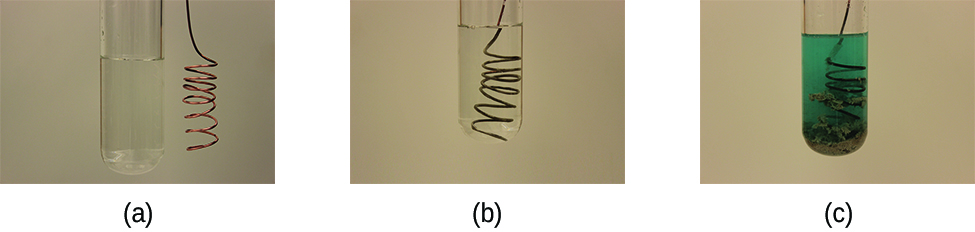
Galvanic or voltaic cells involve spontaneous electrochemical reactions in which the half-reactions are separated (Figure \(\PageIndex{2}\)) so that current can flow through an external wire. The beaker on the left side of the figure is called a half-cell, and contains a 1 M solution of copper(II) nitrate [Cu(NO3)2] with a piece of copper metal partially submerged in the solution. The copper metal is an electrode. The copper is undergoing oxidation; therefore, the copper electrode is the anode. The anode is connected to a voltmeter with a wire and the other terminal of the voltmeter is connected to a silver electrode by a wire. The silver is undergoing reduction; therefore, the silver electrode is the cathode. The half-cell on the right side of the figure consists of the silver electrode in a 1 M solution of silver nitrate (AgNO3). At this point, no current flows—that is, no significant movement of electrons through the wire occurs because the circuit is open. The circuit is closed using a salt bridge, which transmits the current with moving ions.
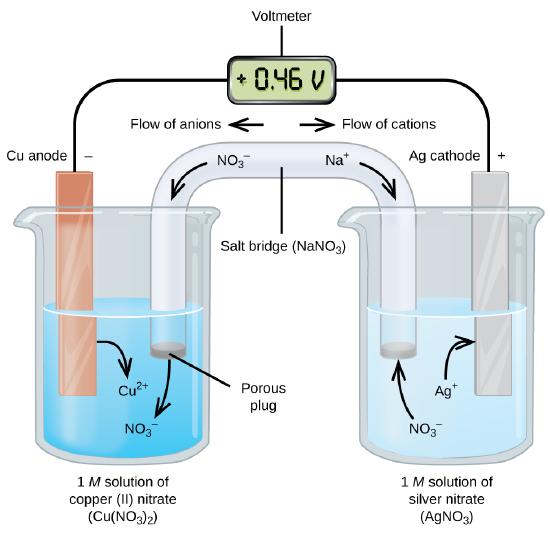
The salt bridge consists of a concentrated, nonreactive, electrolyte solution such as the sodium nitrate (NaNO3) solution used in this example. As electrons flow from left to right through the electrode and wire, nitrate ions (anions) pass through the porous plug on the left into the copper(II) nitrate solution. This keeps the beaker on the left electrically neutral by neutralizing the charge on the copper(II) ions that are produced in the solution as the copper metal is oxidized. At the same time, the nitrate ions are moving to the left, sodium ions (cations) move to the right, through the porous plug, and into the silver nitrate solution on the right. These added cations “replace” the silver ions that are removed from the solution as they were reduced to silver metal, keeping the beaker on the right electrically neutral.
When the electrochemical cell is constructed in this fashion, a positive cell potential (0.46 V) indicates a spontaneous reaction and that the electrons are flowing from the left to the right.
Key Features of a Standard Galvanic Cell (as shown in Figure \(\PageIndex{2}\)
- Electrons flow from the anode to the cathode: left to right in the standard galvanic cell.
- The electrode in the left half-cell is the anode because oxidation occurs here. The name refers to the flow of anions in the salt bridge toward it.
- The electrode in the right half-cell is the cathode because reduction occurs here. The name refers to the flow of cations in the salt bridge toward it.
- Oxidation occurs at the anode (the left half-cell in the figure).
- Reduction occurs at the cathode (the right half-cell in the figure).
- The salt bridge must be present to close (complete) the circuit and both an oxidation and reduction must occur for current to flow.
Example \(\PageIndex{1}\): Chromium-Copper Galvanic Cell
Consider a galvanic cell consisting of
\(\ce{2Cr}(s)+\ce{3Cu^2+}(aq)⟶\ce{2Cr^3+}(aq)+\ce{3Cu}(s)\)
Write the oxidation and reduction half-reactions. Which reaction occurs at the anode? The cathode?
Solution
By inspection, Cr is oxidized when three electrons are lost to form Cr3+, and Cu2+ is reduced as it gains two electrons to form Cu. Balancing the charge gives
\(\begin{align*}
&\textrm{oxidation: }\ce{2Cr}(s)⟶\ce{2Cr^3+}(aq)+\ce{6e-}\\
&\textrm{reduction: }\ce{3Cu^2+}(aq)+\ce{6e-}⟶\ce{3Cu}(s)\\
&\overline{\textrm{overall: }\ce{2Cr}(s)+\ce{3Cu^2+}(aq)⟶\ce{2Cr^3+}(aq)+\ce{3Cu}(s)}
\end{align*}\)
Cell notation uses the simplest form of each of the equations, and starts with the reaction at the anode. No concentrations were specified so:
\(\ce{Cr}(s)│\ce{Cr^3+}(aq)║\ce{Cu^2+}(aq)│\ce{Cu}(s).\)
Oxidation occurs at the anode and reduction at the cathode.
Exercise \(\PageIndex{1}\) Copper-Zinc Galvanic Cell
Consider a galvanic cell consisting of
\(\ce{Zn}(s)+\ce{Cu^2+}(aq)⟶\ce{Zn^2+}(aq)+\ce{Cu}(s)\)
Write the oxidation and reduction half-reactions. Which reaction occurs at the anode? The cathode?
- Answer
-
From the information given in the problem:
\(\begin{align*}
&\textrm{anode (oxidation): }\ce{Zn}(s)⟶\ce{Zn^2+}(aq)+\ce{2e-}\\
&\textrm{cathode (reduction): }\ce{Cu^2+}(aq)+\ce{2e-}⟶\ce{Cu}(s)\\
&\overline{\textrm{overall: }\ce{Zn}(s)+\ce{Cu^2+}(aq)⟶\ce{Zn^2+}(aq)+\ce{Cu}(s)}
\end{align*}\)
Batteries
A battery is an electrochemical cell or series of cells that produces an electric current. In principle, any galvanic cell could be used as a battery. An ideal battery would never run down, produce an unchanging voltage, and be capable of withstanding environmental extremes of heat and humidity. Real batteries strike a balance between ideal characteristics and practical limitations. For example, the mass of a car battery is about 18 kg or about 1% of the mass of an average car or light-duty truck. This type of battery would supply nearly unlimited energy if used in a smartphone, but would be rejected for this application because of its mass. Thus, no single battery is “best” and batteries are selected for a particular application, keeping things like the mass of the battery, its cost, reliability, and current capacity in mind. There are two basic types of batteries: primary and secondary. A few batteries of each type are described next.
Visit this site to learn more about batteries.
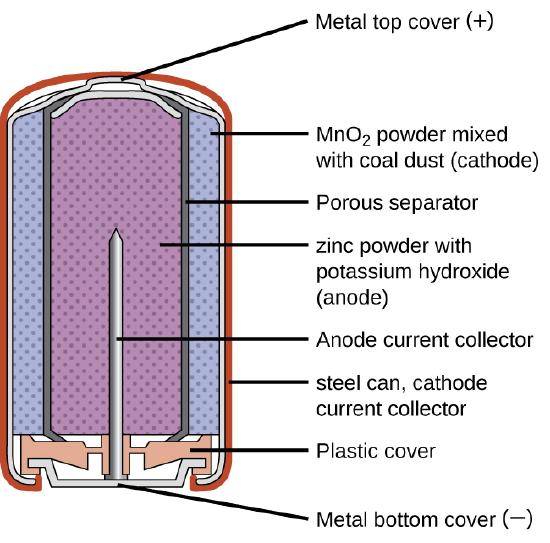
Dry Cells (Primary Batteries)
Primary batteries are single-use batteries because they cannot be recharged. A common primary battery is the dry cell (Figure \(\PageIndex{1}\)). The dry cell is a zinc-carbon battery. The zinc can serves as both a container and the negative electrode. The positive electrode is a rod made of carbon that is surrounded by a paste of manganese(IV) oxide, zinc chloride, ammonium chloride, carbon powder, and a small amount of water. The reaction at the anode can be represented as the ordinary oxidation of zinc:
\[\ce{Zn}(s)⟶\ce{Zn^2+}(aq)+\ce{2e-} \nonumber \]

The reaction at the cathode is more complicated, in part because more than one reaction occurs. The series of reactions that occurs at the cathode is approximately
\[\ce{2MnO2}(s)+\ce{2NH4Cl}(aq)+\ce{2e-}⟶\ce{Mn2O3}(s)+\ce{2NH3}(aq)+\ce{H2O}(l)+\ce{2Cl-} \nonumber \]
The overall reaction for the zinc–carbon battery can be represented as
\[\ce{2MnO2}(s) + \ce{2NH4Cl}(aq) + \ce{Zn}(s) ⟶ \ce{Zn^2+}(aq) + \ce{Mn2O3}(s) + \ce{2NH3}(aq) + \ce{H2O}(l) + \ce{2Cl-} \nonumber \]
with an overall cell potential which is initially about 1.5 V, but decreases as the battery is used. It is important to remember that the voltage delivered by a battery is the same regardless of the size of a battery. For this reason, D, C, A, AA, and AAA batteries all have the same voltage rating. However, larger batteries can deliver more moles of electrons.
Take Note
The dry cell is not very efficient in producing electrical energy because only the relatively small fraction of the \(MnO_2\) that is near the cathode is actually reduced and only a small fraction of the zinc cathode is actually consumed as the cell discharges. In addition, dry cells have a limited shelf life because the \(Zn\) anode reacts spontaneously with \(NH_4Cl\) in the electrolyte, causing the case to corrode and allowing the contents to leak out (Figure \(\PageIndex{2}\)).

Alkaline batteries (Figure \(\PageIndex{4}\)) were developed in the 1950s partly to address some of the performance issues with zinc–carbon dry cells. They are manufactured to be exact replacements for zinc-carbon dry cells. As their name suggests, these types of batteries use alkaline electrolytes, often potassium hydroxide.
An alkaline battery can deliver about three to five times the energy of a zinc-carbon dry cell of similar size. Alkaline batteries are prone to leaking potassium hydroxide, so these should also be removed from devices for long-term storage. While some alkaline batteries are rechargeable, most are not.
Warning
Attempts to recharge an alkaline battery that is not rechargeable often leads to rupture of the battery and leakage of the potassium hydroxide electrolyte.
Lead Storage Batteries (Secondary Batteries)
The lead acid battery (Figure \(\PageIndex{5}\)) is the type of secondary battery used in your automobile. Secondary batteries are rechargeable. The lead acid battery is inexpensive and capable of producing the high current required by automobile starter motors. The reactions for a lead acid battery are
\[\begin{align*} &\textrm{anode: }\ce{Pb}(s)+\ce{HSO4-}(aq)⟶\ce{PbSO4}(s)+\ce{H+}(aq)+\ce{2e-}\\ &\underline{\textrm{cathode: } \ce{PbO2}(s)+\ce{HSO4-}(aq)+\ce{3H+}(aq)+\ce{2e-}⟶\ce{PbSO4}(s)+\ce{2H2O}(l)}\\ &\textrm{overall: }\ce{Pb}(s)+\ce{PbO2}(s)+\ce{2H2SO4}(aq)⟶\ce{2PbSO4}(s)+\ce{2H2O}(l) \end{align*} \nonumber \]
Each cell produces 2 V, so six cells are connected in series to produce a 12-V car battery. Lead acid batteries are heavy and contain a caustic liquid electrolyte, but are often still the battery of choice because of their high current density. The lead acid battery in your automobile consists of six cells connected in series to give 12 V. Their low cost and high current output makes these excellent candidates for providing power for automobile starter motors.
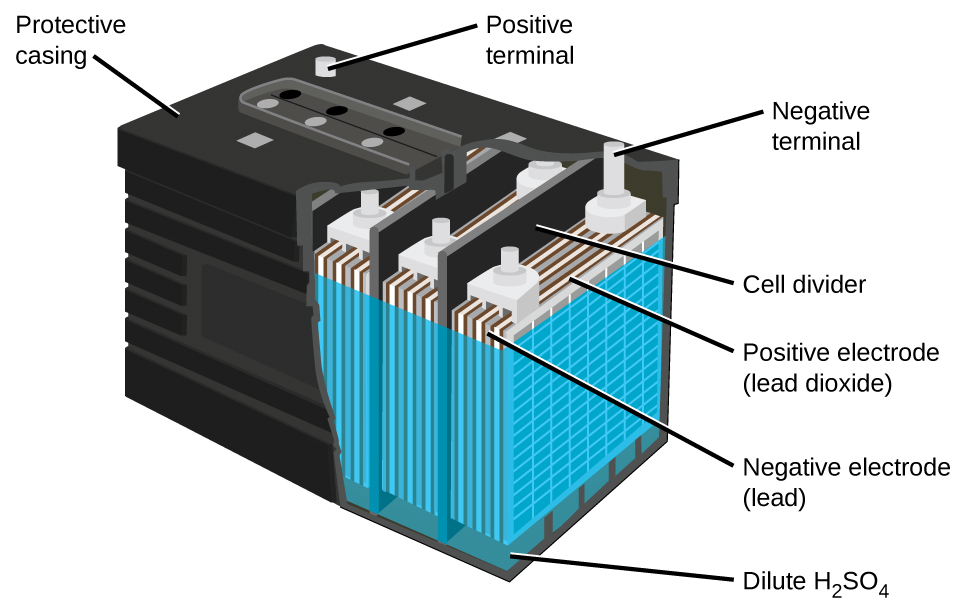
As mentioned earlier, unlike a dry cell, the lead storage battery is rechargeable. Note that the forward redox reaction generates solid lead (II) sulfate which slowly builds up on the plates. Additionally, the concentration of sulfuric acid decreases. When the car is running normally, its generator recharges the battery by forcing the above reactions to run in the opposite, or non spontaneous direction.
\[2 \ce{PbSO_4} \left( s \right) + 2 \ce{H_2O} \left( l \right) \rightarrow \ce{Pb} \left( s \right) + \ce{PbO_2} \left( s \right) + 4 \ce{H+} \left( aq \right) + 2 \ce{SO_4^{2-}} \left( aq \right) \nonumber \]
This reaction regenerates the lead, lead (IV) oxide, and sulfuric acid needed for the battery to function properly. Theoretically, a lead storage battery should last forever. In practice, the recharging is not \(100\%\) efficient because some of the lead (II) sulfate falls from the electrodes and collects on the bottom of the cells.
Warning
Lead storage batteries contain a significant amount of lead so they must always be disposed of properly.
Other Batteries
The nickel–cadmium, or NiCad, battery (Figure \(\PageIndex{6}\)) is used in small electrical appliances and devices like drills,
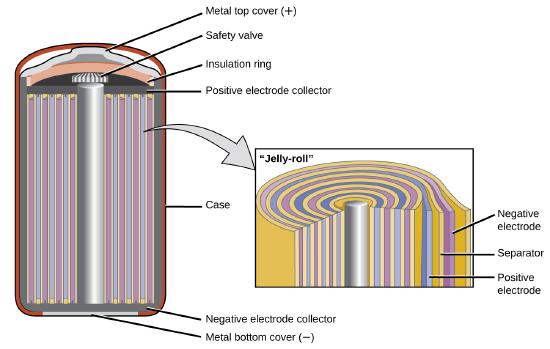
portable vacuum cleaners, and AM/FM digital tuners. It consists of a nickel-plated cathode, cadmium-plated anode, and a potassium hydroxide electrode. The positive and negative plates, which are prevented from shorting by the separator, are rolled together and put into the case. This is a “jelly-roll” design and allows the NiCd cell to deliver much more current than a similar-sized alkaline battery.
The voltage is about 1.2 V to 1.25 V as the battery discharges. When properly treated, a NiCd battery can be recharged about 1000 times.
Warning
Cadmium is a toxic heavy metal so NiCd batteries should never be opened or put into the regular trash.
Lithium ion batteries are among the most popular rechargeable batteries and are used in many portable electronic devices. The battery voltage is about 3.7 V. Lithium batteries are popular because they can provide a large amount current, are lighter than comparable batteries of other types, produce a nearly constant voltage as they discharge, and only slowly lose their charge when stored. Specialized lithium-iodide (polymer) batteries find application in many long-life, critical devices, such as pacemakers and other implantable electronic medical devices. These devices are designed to last 15 or more years.
Disposable primary lithium batteries must be distinguished from secondary lithium-ion or a lithium-polymer. The term "lithium battery" refers to a family of different lithium-metal chemistries, comprising many types of cathodes and electrolytes but all with metallic lithium as the anode. Lithium batteries are widely used in portable consumer electronic devices, and in electric vehicles ranging from full sized vehicles to radio controlled toys.

(0.197 to 0.984 in) in diameter and 1 to 6 mm (0.039 to 0.236 in) high — like a button on a garment, hence the name. A metal can forms the bottom body and positive terminal of the cell. An insulated top cap is the negative terminal. Button cells are single cells, usually disposable primary cells. Common anode materials are zinc or lithium. Common cathode materials are manganese dioxide, silver oxide, carbon monofluoride, cupric oxide or oxygen from the air. Mercuric oxide button cells were formerly common, but are no longer available due to the toxicity and environmental effects of mercury.
Warning
Button cells are very dangerous for small children. Button cells that are swallowed can cause severe internal burns and significant injury or death.
Fuel Cells
A fuel cell is a device that converts chemical energy into electrical energy. Fuel cells are similar to
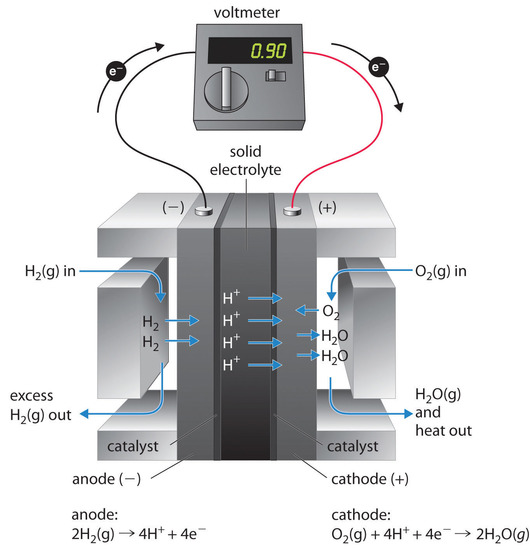
batteries but require a continuous source of fuel, often hydrogen. They will continue to produce electricity as long as fuel is available. Hydrogen fuel cells have been used to supply power for satellites, space capsules, automobiles, boats, and submarines (Figure \(\PageIndex{8}\)).
The voltage is about 0.9 V. The efficiency of fuel cells is typically about 40% to 60%, which is higher than the typical internal combustion engine (25% to 35%) and, in the case of the hydrogen fuel cell, produces only water as exhaust. Although fuel cells are an essentially pollution-free means of obtaining electrical energy, their expense and technological complexity have thus far limited their applications.
Electrolysis
In this chapter, we have described various galvanic cells in which a spontaneous chemical reaction is used to generate electrical energy. In an electrolytic cell, however, the opposite process, called electrolysis, occurs: an external voltage is applied to drive a nonspontaneous reaction. Electrolysis has many commercial and industrial applications. Electrometallurgy is the process of reduction of metals from metallic compounds to obtain the pure form of metal using electrolysis. Aluminium, lithium, sodium, potassium, magnesium, calcium, and in some cases copper, are produced in this way. Chlorine and sodium hydroxide are the major products of the electrolysis of brine, a water/sodium chloride mixture. Water electrolysis is used to generate oxygen for spacecraft and nuclear submarines.
Electrolysis is also used in the cleaning and preservation of old artifacts. Because the process separates the non-metallic particles from the metallic ones, it is very useful for cleaning a wide variety of metallic objects, from old coins to even larger objects including rusted cast iron cylinder blocks and heads when rebuilding automobile engines. Rust removal from small iron or steel objects by electrolysis can be done in a home workshop using simple materials such as a plastic bucket, tap water, lengths of rebar, washing soda, baling wire, and a battery charger.
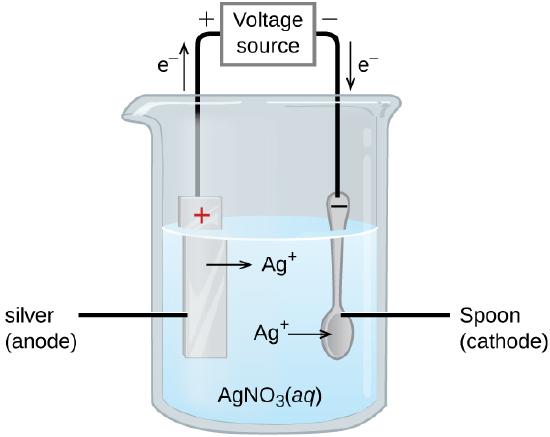
An important use for electrolytic cells is in electroplating. Electroplating results in a thin coating of one metal on top of a conducting surface. Reasons for electroplating include making the object more corrosion resistant, strengthening the surface, producing a more attractive finish, or for purifying metal. The metals commonly used in electroplating include cadmium, chromium, copper, gold, nickel, silver, and tin. Common consumer products include silver-plated or gold-plated tableware, chrome-plated automobile parts, and jewelry. We can get an idea of how this works by investigating how silver-plated tableware is produced (Figure \(\PageIndex{9}\)).
Summary
- Electrochemistry is a branch of chemistry that deals with the interconversion of chemical energy and electrical energy.
- Batteries are galvanic cells, or a series of cells, that produce an electric current.
- There are two basic types of batteries: primary and secondary.
- Primary batteries are “single use” and cannot be recharged. Dry cells and (most) alkaline batteries are examples of primary batteries.
- The second type is rechargeable and is called a secondary battery. Examples of secondary batteries include nickel-cadmium (NiCd), lead acid, and lithium ion batteries.
- Fuel cells are similar to batteries in that they generate an electrical current, but require continuous addition of fuel and oxidizer. The hydrogen fuel cell uses hydrogen and oxygen from the air to produce water, and is generally more efficient than internal combustion engines.
- Electrolysis occurs when an external voltage is applied to drive a nonspontaneous reaction.
Contributors and Attributions
Paul Flowers (University of North Carolina - Pembroke), Klaus Theopold (University of Delaware) and Richard Langley (Stephen F. Austin State University) with contributing authors. Textbook content produced by OpenStax College is licensed under a Creative Commons Attribution License 4.0 license. Download for free at http://cnx.org/contents/85abf193-2bd...a7ac8df6@9.110).
- TextMap: Chemistry-The Central Science (Brown et al.)
- Wikipedia

