20.8: Synthesis using nucleophilic addition chemistry
- Page ID
- 225895
Carbon-carbon bond forming reactions
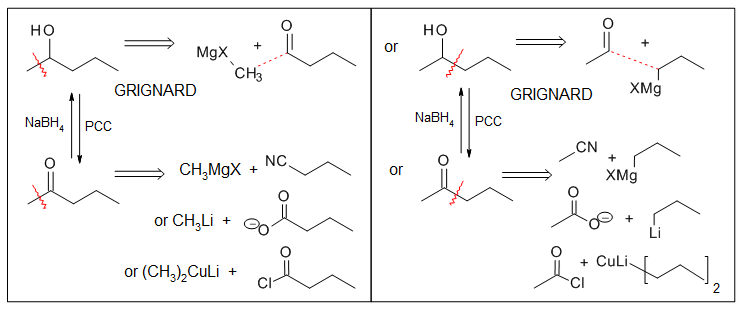
Organometallics and Wittig reactions in synthesis
One important route for producing an alcohol from a Grignard reagent has been omitted from the discussion in the reading. It involves the reaction of the Grignard reagent with ethylene oxide to produce a primary alcohol containing two more carbon atoms than the original Grignard reagent.
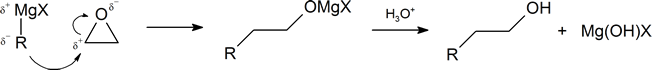
As mentioned in the reading, both organolithium and Grignard reagents are good nucleophiles. They also act as strong bases in the presence of acidic protons such as −CO2H, −OH, −SH, −NH and terminal alkyne groups. Not only do acidic protons interfere with the nucleophilic attack on the carbonyl of these organometallic reagents, if the starting materials possess any acidic protons, reagents cannot be generated in the first place. They are also the reason these reactions must be carried out in a water‑free environment.
Another limitation of preparing Grignard and organolithium reagents is that they cannot already contain a carbonyl group (or other electrophilic multiple bonds like C$\ce{=}$N C$\ce{#}$N, N$\ce{=}$O S$\ce{=}$O) because it would simply react with itself.
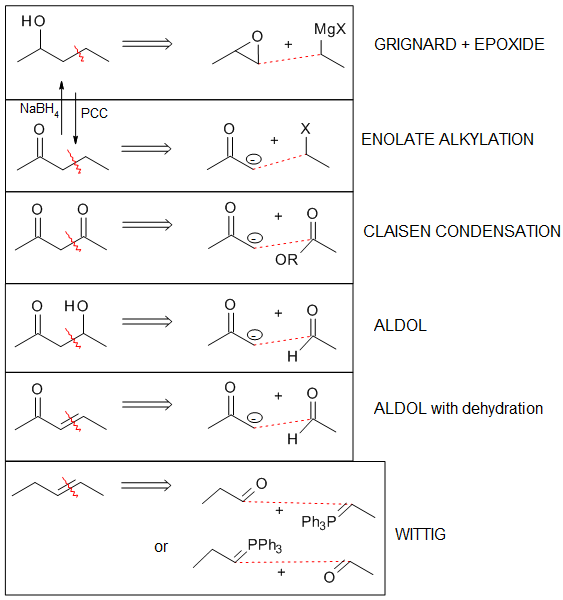
A summary of the methods used to prepare alcohols from Grignard reagents is provided below.
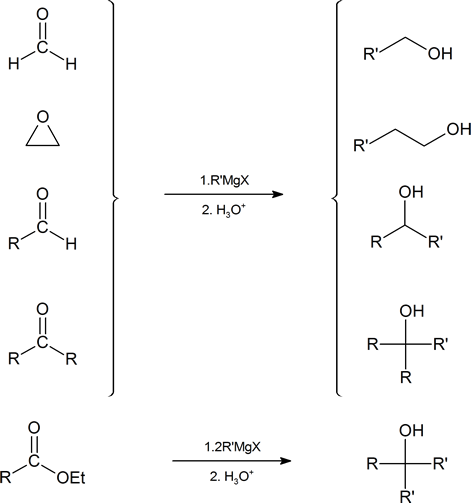
Many of these alcohols can be converted to an alkene, by heating with an acid such as H2SO4 or H3PO4., via an E1 elimination (see section 9.9.). However, this can often give mixtures of isomeric alkene products with the double bond at different positions. Therefore, when the target product of a C-C bond forming reaction is an alkene, it is usually better to use a Wittig reaction instead, because this will form the new double bond unambiguously at one position.
Using the aldol reaction in synthesis
Objectives
After completing this section, you should be able to identify the aldehyde or ketone and other necessary reagents that should be used to prepare a given enone by an aldol condensation.
Aldol reactions are excellent methods for the synthesis of many enones or beta hydroxy carbonyls. Because of this, being able to predict when an aldol reaction might be used in a synthesis in an important skill. This accomplished by mentally breaking apart the target molecule and then considering what the starting materials might be.
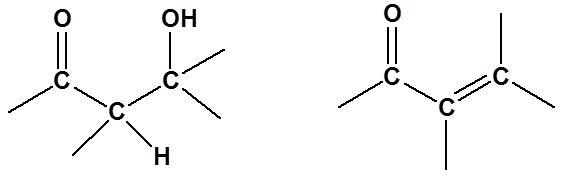
Alpha,beta-unsaturated carbonyl compounds, such as the enone shown above, are useful for performing conjugate addition reactions, as was covered in section 20.7.
- Synthesis using nucleophilic addition chemistry. Authored by: Martin A. Walker. Provided by: SUNY Potsdam. Project: Organic chemistry: An open textbook. License: CC BY-SA: Attribution-ShareAlike
- Organic Chemistry With a Biological Emphasis . Authored by: Tim Soderberg. Located at: https://chem.libretexts.org/Textbook_Maps/Organic_Chemistry/Book%3A_Organic_Chemistry_with_a_Biological_Emphasis_(Soderberg)/13%3A_Reactions_with_stabilized_carbanion_intermediates_I/13.6%3A_Synthetic_parallel_-_carbon_nucleophiles_in_the_lab#13.6D:_Grignard.2C_Gilman.2C_and_organolithiuim_reagents. Project: Chemistry LibreTexts. License: CC BY-NC-SA: Attribution-NonCommercial-ShareAlike

