23.3: Reactions of amines
- Page ID
- 225907
Amide formation
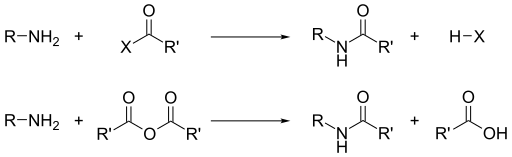
Acyl chlorides and acid anhydrides react with primary and secondary amines without the presence of heat to form amides. Tertiary amines cannot be acylated due to the absence of a replaceable hydrogen atom. With the much less active benzoyl chloride, acylation can still be performed by the use of excess aqueous base to facilitate the reaction.
Salt formation
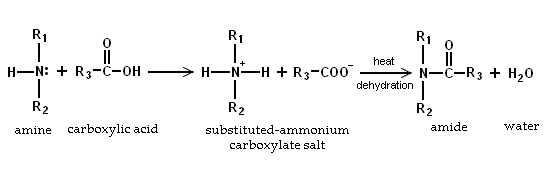
Because amines are basic, they neutralize carboxylic acids to form the corresponding ammonium carboxylate salts. Upon heating to 200°C, the primary and secondary amine salts dehydrate to form the corresponding amides.
Neutralization
Amines R3N react with strong acids such as hydroiodic acid (HI), hydrobromic acid (HBr) and hydrochloric acid (HCl) to give ammonium salts R3NH+.
Reaction with nitrous acid
Nitrous acid with the chemical formula HNO2 is unstable. Usually it is produced indirectly in a mixture of NaNO2 and a strong acid such as HCl or H2SO4 in dilute concentration, so that the H+ ions will associate with the NO2– ions in solution.
Primary aliphatic amines with nitrous acid give very unstable diazonium salts which spontaneously decompose by losing N2 to form a carbenium ion. The carbenium ion goes on to produce a mixture of alkenes, alkanols or alkyl halides, with alkanols as the major product. This reaction is of little synthetic importance because the diazonium salt formed is too unstable, even under quite cold conditions.
- NaNO2 + HCl → HNO2 + NaCl
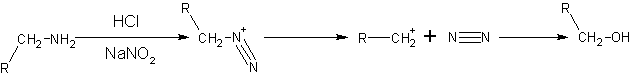
- Primary aromatic amines, such as aniline (phenylamine) forms a more stable diazonium ion at 0–5°C. Above 5°C, it will decompose to give phenol and N2. Diazonium salts can be isolated in the crystalline form but are usually used in solution and immediately after preparation, due to rapid decomposition on standing even with little ambient heat. Solid diazonium salts can be explosive on shock or on mild warming.
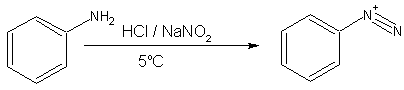
Reactions with ketones and aldehydes
- Primary amines react with carbonyl compounds to form imines (see section 21.4.). Specifically, aldehydes become aldimines, and ketones become ketimines. In the case of formaldehyde (R’ = H), the imine products are typically cyclic trimers.
- RNH2 + R’2C=O → R’2C=NR + H2O
- Secondary amines react with ketones and aldehydes to form enamines. An enamine contains a C=C double bond, where the second C is singly bonded to N as part of an amine ligand.
- R2NH + R'(R”CH2)C=O → R”CH=C(NR2)R’ + H2O
- Organic Chemistry/Amines. Authored by: Wikibooks contributors. Provided by: Wikibooks. Located at: https://en.wikibooks.org/wiki/Organic_Chemistry/Amines. Project: Book: Organic Chemistry. License: CC BY-SA: Attribution-ShareAlike

