19.3: Reductions using NaBH4, LiAlH4
- Page ID
- 225882
Reduction of aldehydes and ketones
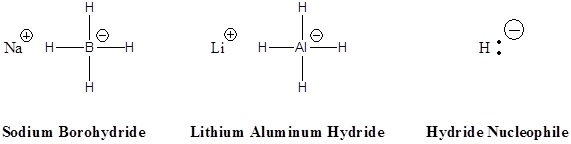 Addition of a hydride anion (H:–) to an aldehyde or ketone gives an alkoxide anion, which on protonation yields the corresponding alcohol. Aldehydes produce 1º-alcohols and ketones produce 2º-alcohols.
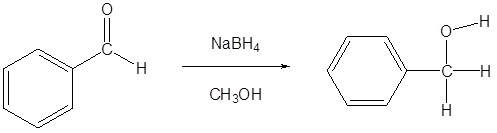
Addition of a hydride anion (H:–) to an aldehyde or ketone gives an alkoxide anion, which on protonation yields the corresponding alcohol. Aldehydes produce 1º-alcohols and ketones produce 2º-alcohols.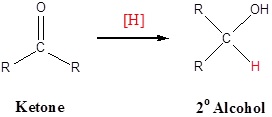 In metal hydrides reductions the resulting alkoxide salts are insoluble and need to be hydrolyzed (with care) before the alcohol product can be isolated. In the sodium borohydride reduction the methanol solvent system achieves this hydrolysis automatically. In the lithium aluminium hydride reduction water is usually added in a second step. The lithium, sodium, boron and aluminium end up as soluble inorganic salts at the end of either reaction. Note! LiAlH4 and NaBH4 are both capable of reducing aldehydes and ketones to the corresponding alcohol.
In metal hydrides reductions the resulting alkoxide salts are insoluble and need to be hydrolyzed (with care) before the alcohol product can be isolated. In the sodium borohydride reduction the methanol solvent system achieves this hydrolysis automatically. In the lithium aluminium hydride reduction water is usually added in a second step. The lithium, sodium, boron and aluminium end up as soluble inorganic salts at the end of either reaction. Note! LiAlH4 and NaBH4 are both capable of reducing aldehydes and ketones to the corresponding alcohol. Examples

Mechanism
This mechanism is for a LiAlH4 reduction. The mechanism for a NaBH4 reduction is the same except methanol is the proton source used in the second step.
1) Nucleophilic attack by the hydride anion
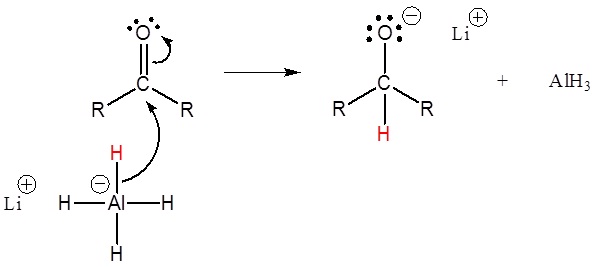
2) The alkoxide is protonated
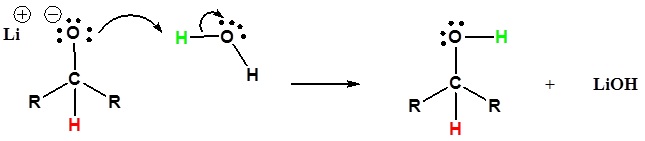
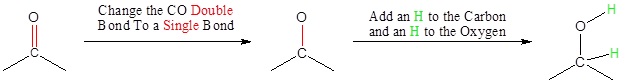
Going from Reactants to Products Simplified
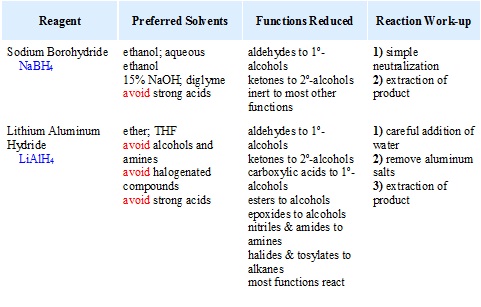
Properties of hydride sources
Two practical sources of hydride-like reactivity are the complex metal hydrides lithium aluminium hydride (LiAlH4) and sodium borohydride (NaBH4). These are both white (or near white) solids, which are prepared from lithium or sodium hydrides by reaction with aluminum or boron halides and esters. Lithium aluminium hydride is by far the more reactive of the two compounds, reacting violently with water, alcohols and other acidic groups with the evolution of hydrogen gas. The following table summarizes some important characteristics of these useful reagents.
Exercises
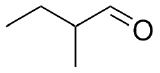
1) Please draw the products of the following reactions:
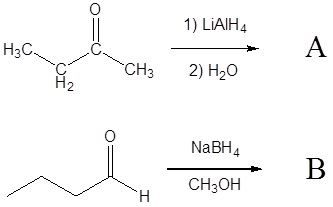
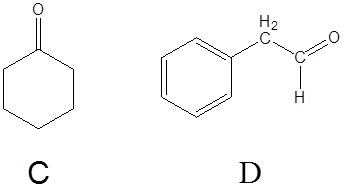
2) Please draw the structure of the molecule which must be reacted to produce the product.
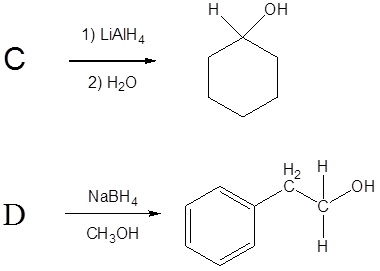
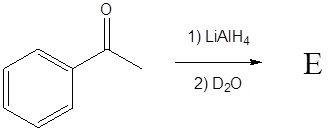
3) Deuterium oxide (D2O) is a form of water where the hydrogens have been replaced by deuteriums. For the following LiAlH4 reduction the water typically used has been replaced by deuterium oxide. Please draw the product of the reaction and place the deuterium in the proper location. Hint! Look at the mechanism of the reaction.
Answers
[reveal-answer q=”534587″]Show Answer[/reveal-answer]
[hidden-answer a=”534587″]
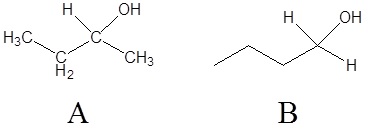
1)
2)
3)
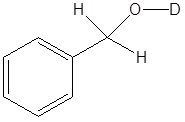
[/hidden-answer]
Contributors
- Prof. Steven Farmer (Sonoma State University)
- William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry
Alcohols from carbonyl compounds: Reduction
Aldehydes, ketones and alcohols are very common features in biological molecules. Converting between these compounds is a frequent event in many biological pathways. However, semi-anionic compounds like sodium borohydride don’t exist in the cell. Instead, a number of biological hydride donors play a similar role.
NADH is a common biological reducing agent. NADH is an acronym for nicotinamide adenine dinucleotide hydride. Insetad of an anionic donor that provides a hydride to a carbonyl, NADH is actually a neutral donor. It supplies a hydride to the carbonyl under very specific circumstances. In doing so, it forms a cation, NAD+. However, NAD+ is stabilized by the fact that its nicotinamide ring is aromatic; it was not aromatic in NADH.
Reduction of carboxylic acids and esters
Carboxylic acids can be converted to 1o alcohols using Lithium aluminium hydride (LiAlH4). Note that NaBH4 is not strong enough to convert carboxylic acids or esters to alcohols. An aldehyde is produced as an intermediate during this reaction, but it cannot be isolated because it is more reactive than the original carboxylic acid.
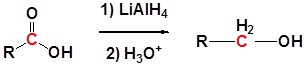
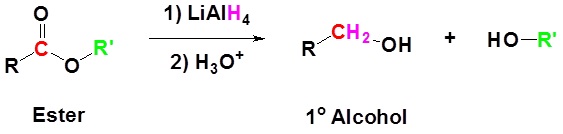
Esters can be converted to 1o alcohols using LiAlH4, while sodium borohydride ($$NaBH_4$$) is not a strong enough reducing agent to perform this reaction.
Exercises
Contributors
- Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
- Prof. Steven Farmer (Sonoma State University)
- Chris P Schaller, Ph.D., (College of Saint Benedict / Saint John’s University)
- Reduction of Aldehydes and Ketones. Authored by: Prof. Steven Farmer, William Reusch. Located at: https://chem.libretexts.org/Textbook_Maps/Organic_Chemistry/Map%3A_Organic_Chemistry_(Smith)/Chapter_20%3A_Introduction_to_Carbonyl_Chemistry%3B_Organometallic_Reagents%3B_Oxidation_and_Reduction/20.04_Reduction_of_Aldehydes_and_Ketones. Project: Chemistry LibreTexts. License: CC BY-NC-SA: Attribution-NonCommercial-ShareAlike
- Alcohols from Carbonyl Compounds: Reduction. Authored by: Dr. Dietmar Kennepohl, Prof. Steven Farmer, Chris P Schaller, Ph.D.. Located at: https://chem.libretexts.org/Textbook_Maps/Organic_Chemistry/Map%3A_Organic_Chemistry_(Smith)/Chapter_20%3A_Introduction_to_Carbonyl_Chemistry%3B_Organometallic_Reagents%3B_Oxidation_and_Reduction/20.04_Reduction_of_Aldehydes_and_Ketones. Project: Chemistry LibreTexts. License: CC BY-NC-SA: Attribution-NonCommercial-ShareAlike


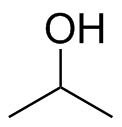 (b)
(b) 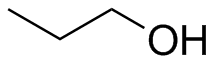 (c)
(c) 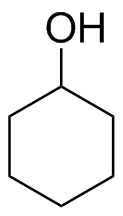 (d)
(d) 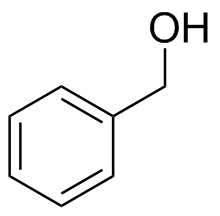
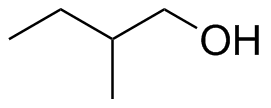 (b)
(b)  (c)
(c)  (d)
(d) 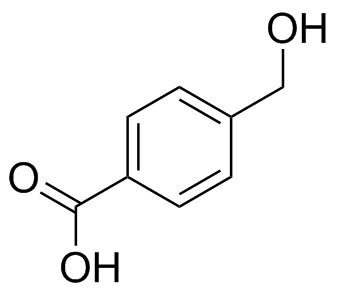
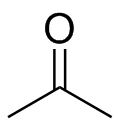 (b)
(b) 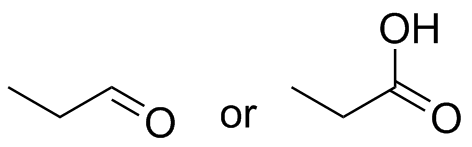 (c)
(c) 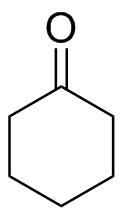 (d)
(d) 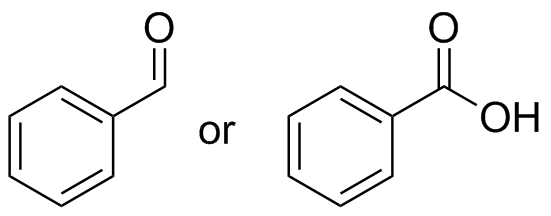
 (b)
(b) 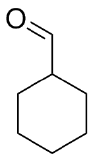 (c)
(c) 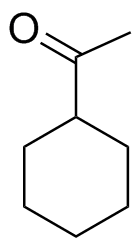 (d)
(d)