18.1: What is a free radical?
- Page ID
- 225875
History
The first organic free radical identified was triphenylmethyl radical. This species was discovered by Moses Gomberg in 1900 at the University of Michigan USA. Historically, the term radical in radical theory was also used for bound parts of the molecule, especially when they remain unchanged in reactions. These are now called functional groups. For example, methyl alcohol was described as consisting of a methyl “radical” and a hydroxyl “radical”. Neither are radicals in the modern chemical sense, as they are permanently bound to each other, and have no unpaired, reactive electrons; however, they can be observed as radicals in mass spectrometry when broken apart by irradiation with energetic electrons.
Depiction in chemical reactions
In chemical equations, free radicals are frequently denoted by a dot placed immediately to the right of the atomic symbol or molecular formula as follows:
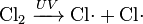
- Chlorine gas can be broken down by ultraviolet light to form atomic chlorine radicals.
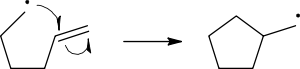
Radical reaction mechanisms use single-headed arrows to depict the movement of single electrons:
The homolytic cleavage of the breaking bond is drawn with a ‘fish-hook’ arrow to distinguish from the usual movement of two electrons depicted by a standard curly arrow. It should be noted that the second electron of the breaking bond also moves to pair up with the attacking radical electron; this is not explicitly indicated in this case.
Free radicals also take part in radical addition and radical substitution as reactive intermediates. Chain reactions involving free radicals can usually be divided into three distinct processes. These are initiation, propagation, and termination., discussed in detail in section 18.4.
- Radical (chemistry). Authored by: Wikipedia contributors. Provided by: Wikimedia Foundation. Located at: https://en.wikipedia.org/wiki/Radical_(chemistry). Project: Wikipedia. License: CC BY-SA: Attribution-ShareAlike