7.6: Regiochemistry of SN1 Reactions with Allylic Electrophiles
- Page ID
- 234563
\(S_N1\) reactions with allylic electrophiles can often lead to more than one possible regiochemical outcome - resonance delocalization of the carbocation intermediate means that more than one carbon is electrophilic. For example, hydrolysis of this allylic alkyl bromide leads to a mixture of primary and secondary allylic alcohols.
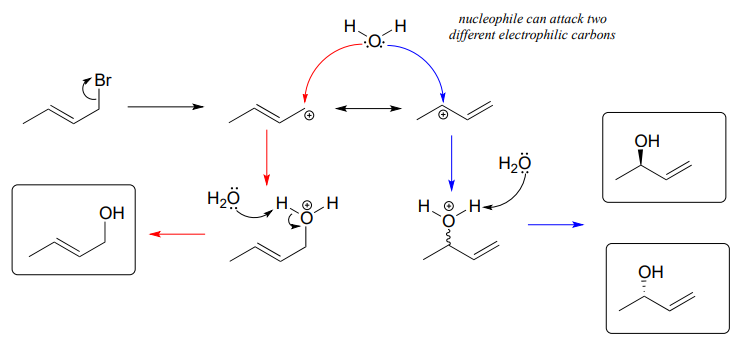
In an enzyme-catalyzed reaction of this kind, however, generally only one product will form, because enzymes maintain strict control over the regiochemistry and stereochemistry of the reactions they catalyze. The nucleophilic and electrophilic substrates are bound specifically in the active site so that nucleophilic attack is directed at one - and only one - electrophilic carbon. Problem 15, 17, and 19 at the end of this chapter provide some examples of regio- and stereospecific biochemical substitution reactions at allylic carbon electrophiles.
Contributors
Organic Chemistry With a Biological Emphasis by Tim Soderberg (University of Minnesota, Morris)


