Solutions to selected Chapter 1 problems
P1.1: The figure below illustrates a section of an intermediate compound that forms during the protein synthesis process in the cell. Lone pairs are not shown, as is typical in drawings of organic compounds.
a) The structure as drawn is incomplete, because it is missing formal charges - fill them in.
b) How many hydrogen atoms are on this structure?
c) Identify the two important biomolecule classes (covered in section 1.3) in the structure.

P1.2: Find, in Table 6 ('Structures of common coenzymes', in the tables section at the back of this book), examples of the following:
a) a thiol b) an amide c) a secondary alcohol d) an aldehyde
e) a methyl substituent on a ring f) a primary ammonium ion
g) a phosphate anhydride h) a phosphate ester
P1.3: Draw line structures corresponding to the following compounds. Show all lone pair electrons (and don't forget that non-zero formal charges are part of a correctly drawn structure!)
a) 2,2,4-trimethylpentane b) 3-phenyl-2-propenal
c) 6-methyl-2,5-cyclohexadienone d) 3-methylbutanenitrile
e) 2,6-dimethyldecane f) 2,2,5,5-tetramethyl-3-hexanol
g) methyl butanoate h) N-ethylhexanamide
i) 7-fluoroheptanoate j) 1-ethyl-3,3-dimethylcyclohexene
P1.4: Reaction A below is part of the biosynthetic pathway for the amino acid methionine, and reaction B is part of the pentose phosphate pathway of sugar metabolism.
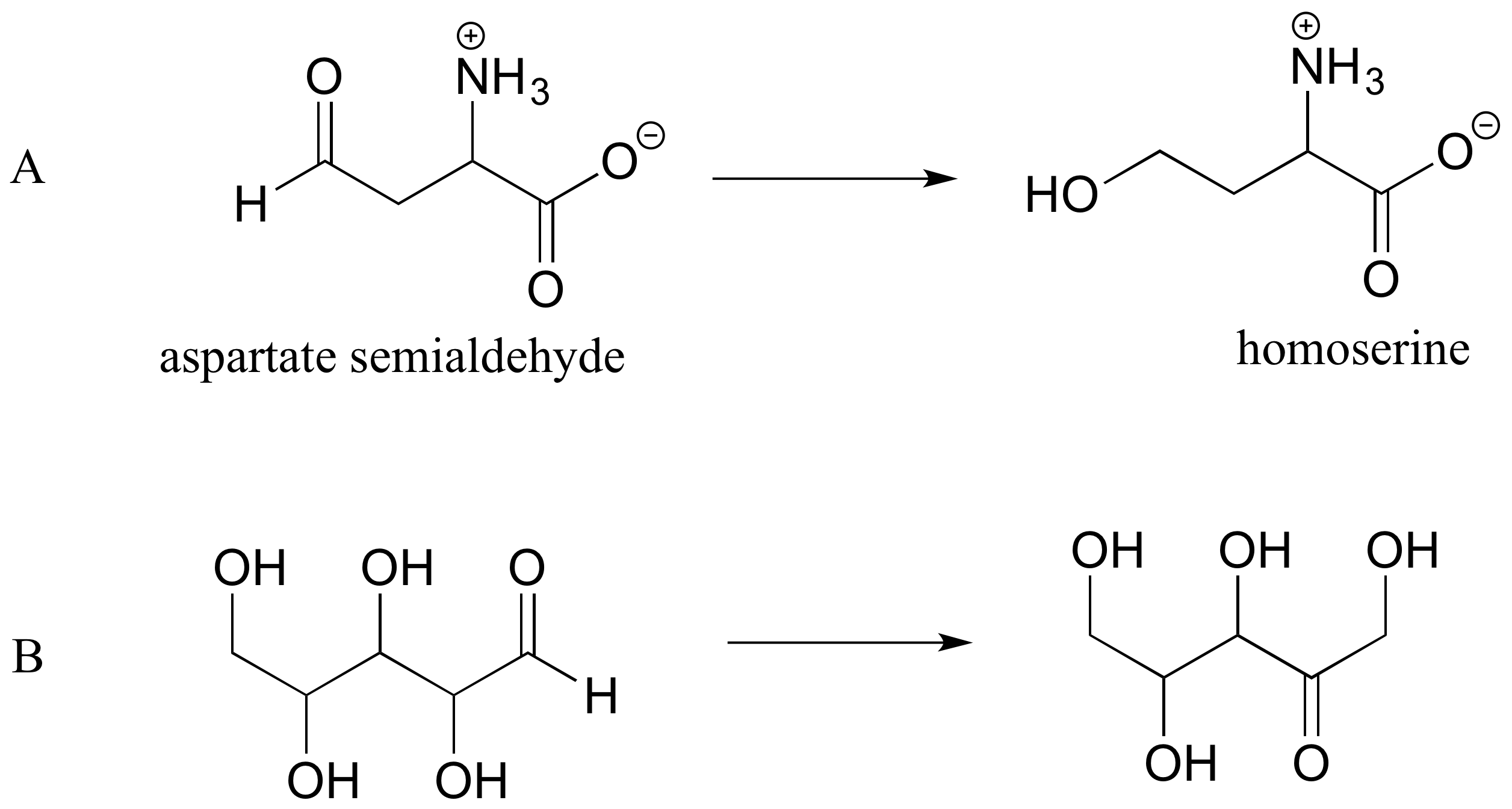
a) What is the functional group transformation that is taking place in each reaction?
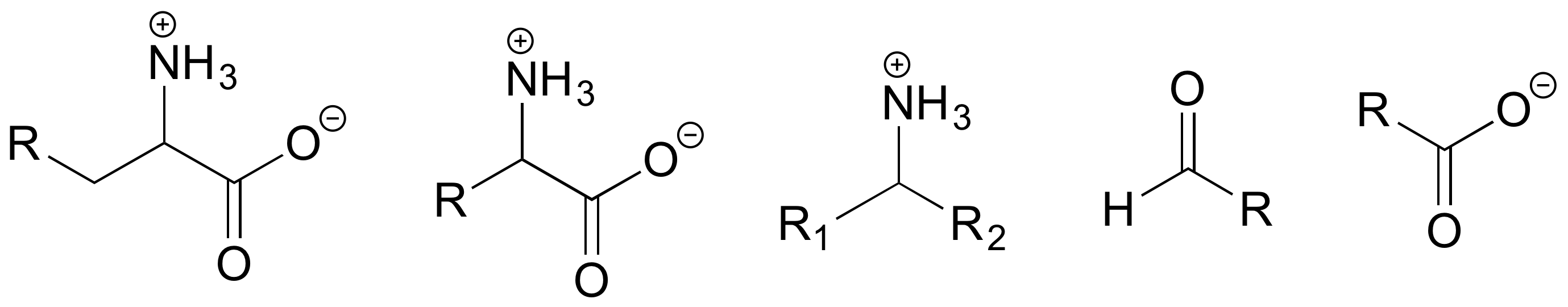
b) Keeping in mind that the 'R' abbreviation is often used to denote parts of a larger molecule which are not the focus of a particular process, which of the following abbreviated structures could be appropriate to use for aspartate semialdehyde when drawing out details of reaction A?
c) Again using the 'R' convention, suggest an appropriate abbreviation for the reactant in reaction B.
P1.5: Find, in the table of amino acid structures (Table 5), examples of the following:
a) a secondary alcohol b) an amide c) a thiol
d) a sulfide e) a phenol f) a side chain primary ammonium
g) a side chain carboxylate h) a secondary amine
P1.6: Draw correct Lewis structures for ozone (O3), azide ion, (N3-), and bicarbonate ion, HCO3-. Include lone pair electrons and formal charges, and use your General Chemistry textbook to review VSEPR theory, which will enable you to draw correct bond geometries.
P1.7: Draw one example each of compounds fitting the descriptions below, using line structures. Be sure to include all non-zero formal charges. All atoms should fit one of the common bonding patters discussed in this chapter. There are many possible correct answers - be sure to check your drawings with your instructor or tutor.
a) an 8-carbon molecule with secondary alcohol, primary amine, amide, and cis-alkene groups
b) a 12-carbon molecule with carboxylate, diphosphate, and lactone (cyclic ester) groups.
c) a 9-carbon molecule with cyclopentane, alkene, ether, and aldehyde groups
P1.8: Three of the four structures below are missing formal charges.

a) Fill in all missing formal charges (assume all atoms have a complete octet of valence electrons).
b) Identify the following functional groups or structural elements (there may be more than one of each): carboxylate, carboxylic acid, cyclopropyl, amide, ketone, secondary ammonium ion, tertiary alcohol.
c) Determine the number of hydrogen atoms in each compound.
P1.9:
a) Draw four constitutional isomers with the molecular formula C4H8. (
b) Draw two open-chain (non-cyclic) constitutional isomers of cyclohexanol (there are more than two possible answers).
P1.10: Draw structures of four different amides with molecular formula C3H7NO.
Solutions to selected Chapter 1 problems
Organic Chemistry With a Biological Emphasis by Tim Soderberg (University of Minnesota, Morris)






