Atoms, Elements, Compounds, and Ions
- Page ID
- 184174
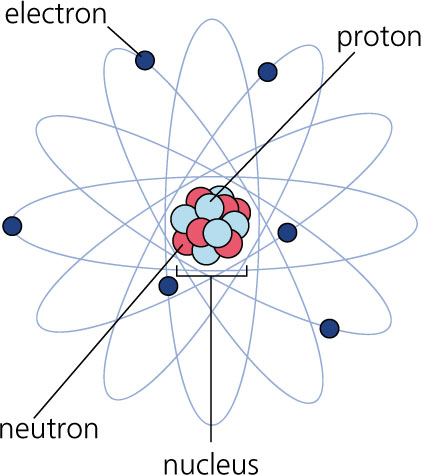 |
Living things are made of atoms of different elements. An atom is the smallest basic unit of matter. An element is made of one type of atom. A few familiar elements might include oxygen, carbon, or hydrogen. Each atom has a nucleus containing protons and neutrons, and electrons that are outside of the nucleus. We won't go too deep into the structure of an atom here, that will be covered in chemistry. What makes one element different from another? Atoms of different elements have different numbers of protons. |
|
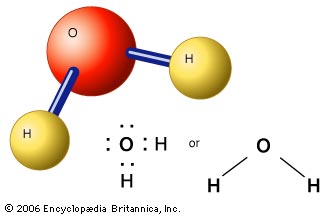
A compound is made of atoms of different elements that are bonded together. An example of a compound is water. To the right you can see three different ways of graphically representing water or H2O. |
|
|
|
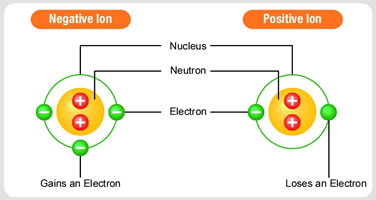
Ions form when an atom gains or loses one or more electrons, this gives the atom a charge. There are both positive ions and negative ions. |