3.4.2. Conformations of Ethane
- Page ID
- 33760
The simple alkane ethane provides a good introduction to conformational analysis. Here there is only one carbon-carbon bond, and the rotational structures (rotamers) that it may assume fall between two extremes, staggered and eclipsed. In the following description of these conformers, several structural notations are used. The first views the ethane molecule from the side, with the carbon-carbon bond being horizontal to the viewer. The hydrogens are then located in the surrounding space by wedge (in front of the plane) and hatched (behind the plane) bonds. If this structure is rotated so that carbon #1 is canted down and brought closer to the viewer, the "sawhorse" projection is presented. Finally, if the viewer looks down the carbon-carbon bond with carbon #1 in front of #2, the Newman projection is seen.
Name of Conformer Wedge-Hatched Bond Structure Sawhorse Structure Newman Projection
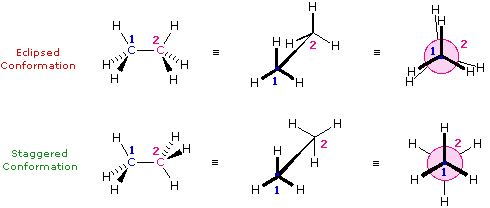
Figure 1: Extreme Conformations of Ethane
As a result of bond-electron repulsions, illustrated in Figure 2, the eclipsed conformation is less stable than the staggered conformation by roughly 3 kcal / mol (eclipsing strain). The most severe repulsions in the eclipsed conformation are depicted by the red arrows. There are six other less strong repulsions that are not shown. In the staggered conformation there are six equal bond repulsions, four of which are shown by the blue arrows, and these are all substantially less severe than the three strongest eclipsed repulsions.
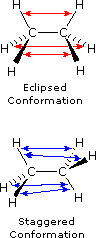
Figure 2: Bond Repulsions in Ethane
Consequently, the potential energy associated with the various conformations of ethane varies with the dihedral angle of the bonds, as shown below. Although the conformers of ethane are in rapid equilibrium with each other, the 3 kcal/mol energy difference leads to a substantial preponderance of staggered conformers (> 99.9%) at any given time.
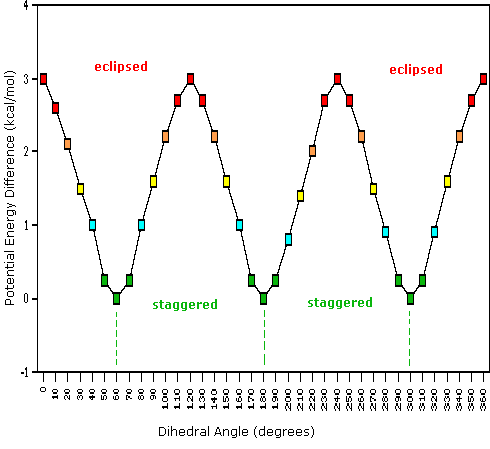
Dihedral Angle
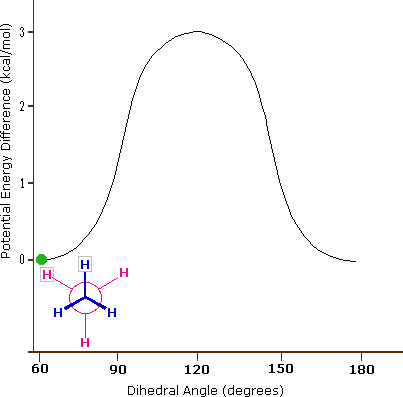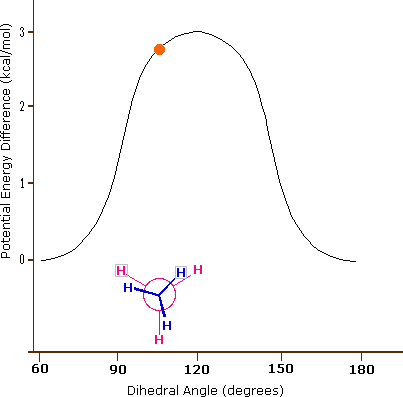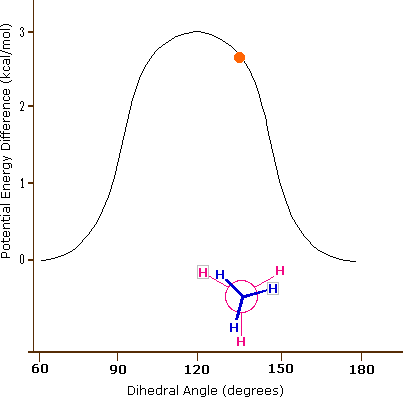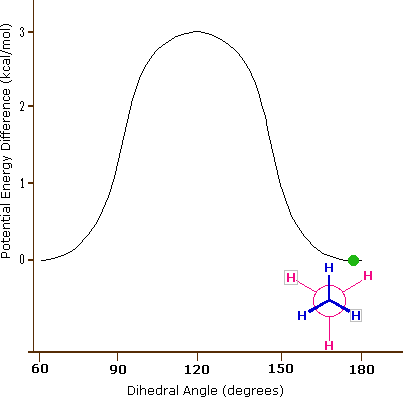
Potential Energy Profile for Ethane Conformers: The above animation illustrates the relationship between ethane's potential energy and its dihedral angle
Contributors
- William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry
Further Reading
Michigan State Virtual Textbook of Organic Chemistry
MasterOrganicChemistry
Carey 4th Edition On-Line Activity
Conformations of simple alkanes
Khan Academy

