11.3: Cycloaddition Reactions of Alkynes
- Page ID
- 321644
Let’s now consider some of the other reactions of alkenes (where \(π_{C-C}\) is the HOMO) and how they might be applied to alkynes.
A. [2+1] cycloadditions
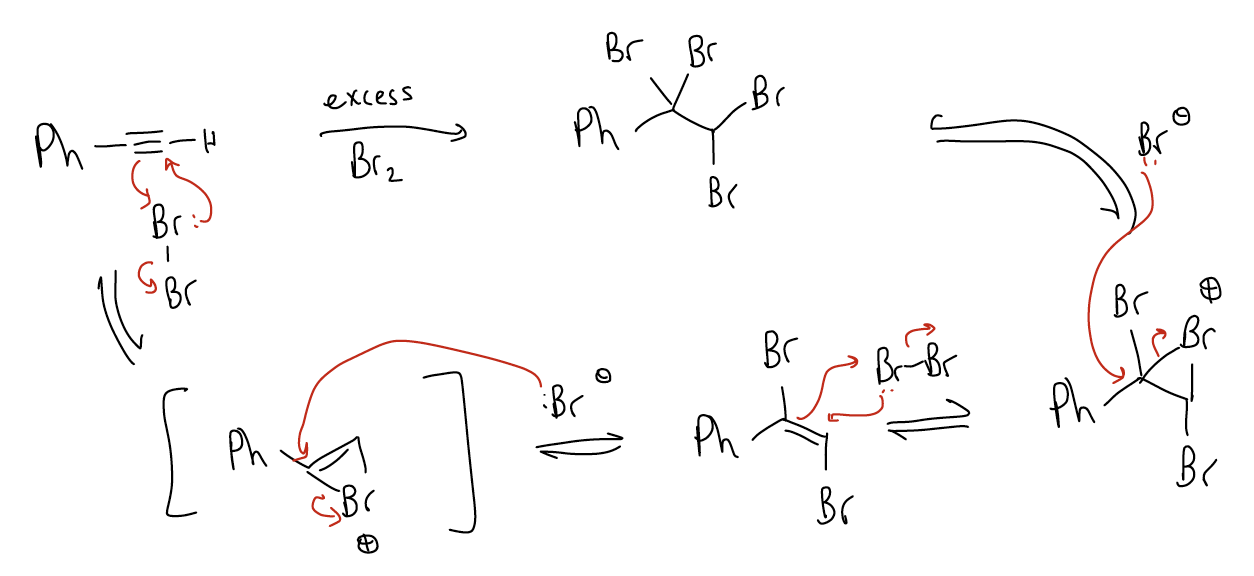
1. Halogenation – This will in formation of a tetrahalide, as shown below for a tetrabromide. The mechanism is quite complex but is thought to proceed via a [2+1] cycloaddition of the alkyne to give a bridged bromonium ion intermediate that contains a double bond – a highly reactive intermediate. Attack of the bromide on the more highly substituted carbon will generate a trans-dibromoalkene. Since this alkene is also reactive towards bromine, a second bromonium ion will form, followed by SN2 to give the final tetrabromide product. It is very difficult to stop the reactive at the trans-dibromoalkene, even when one equivalent is used. Thus, we normally run this reaction in excess bromine.
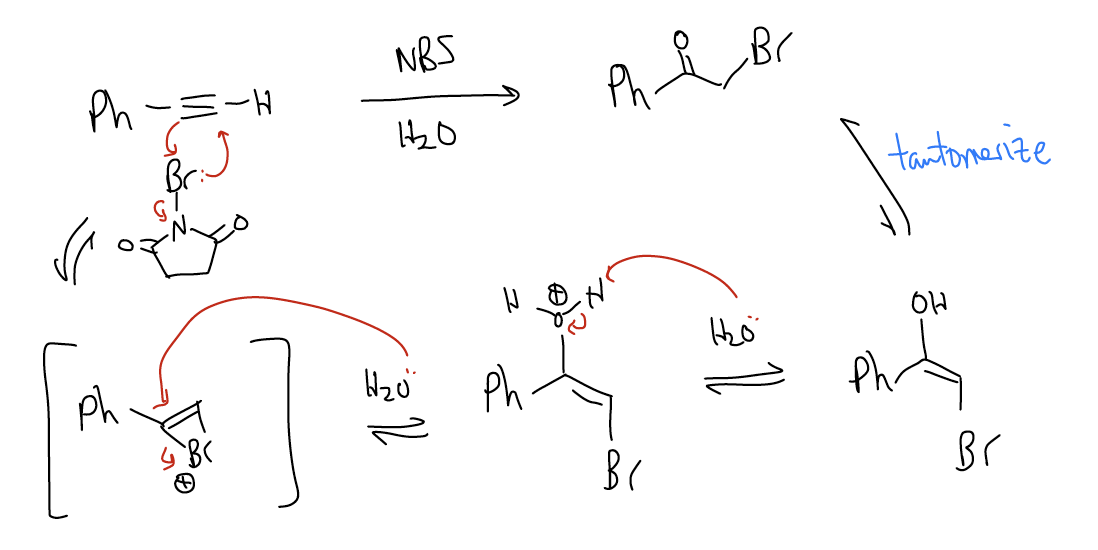
2. Halohydration – This will result in the formation of an \(α\)-haloketone formed by addition of water and a halogen across the triple bond. Using NBS/H2O as an example, initial formation of a bridged bromonium ion will occur via [2+1] cycloaddition. Attack of this species by water will generate an enol upon deprotonation. This enol will tautomerize to the keto form, which contains a bromide at the \(α\)-position. Thus, halohydration is a good method of creating an \(α\)-haloketone, which rapidly undergoes SN2 chemistry.
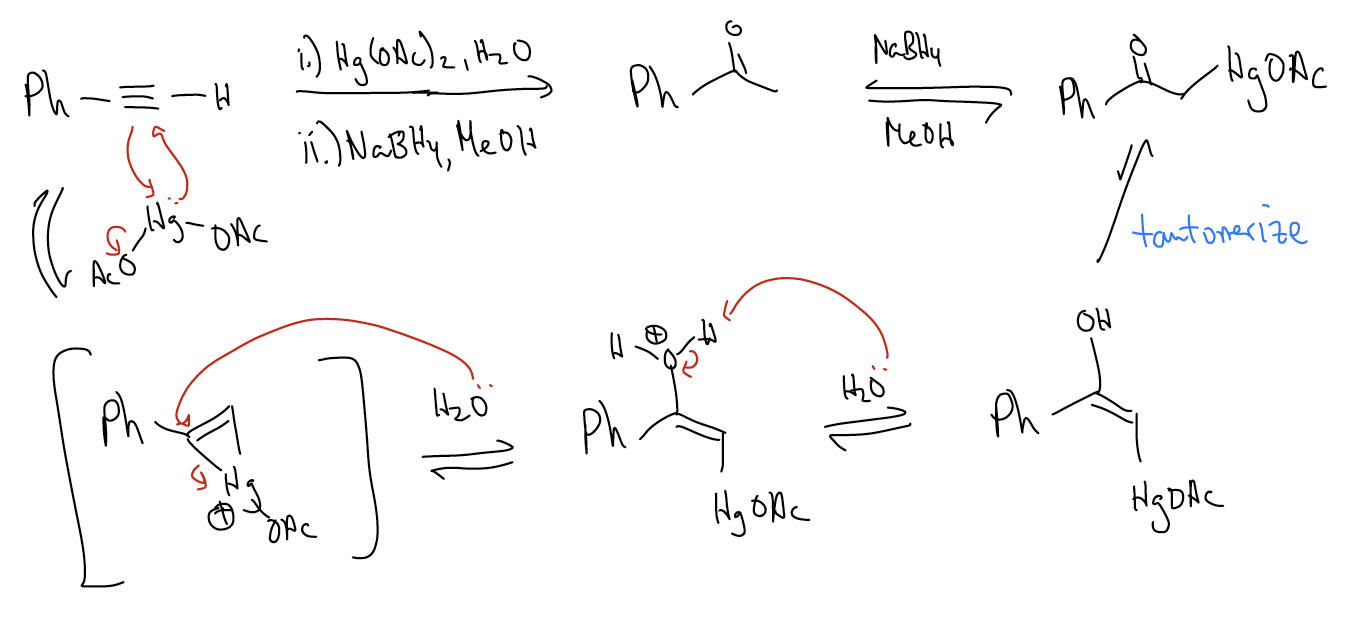
3. Oxymercuration – This will result in the formation of a ketone, formed by Markovnikov addition of water across the triple bond. Once again, formation of a mercurium ion will occur via a [2+1] cycloaddition. Water will then attack the \(σ_{C-Hg}\) orbital, resulting in formation of a enol upon deprotonation. Sodium borohydride (NaBH4) will then reduce the \(σ_{C-Hg]}\) bond to a \(σ_{C-H]}\) bond. The enol will tautomerize to the keto form.
B. [2+2] cycloadditions
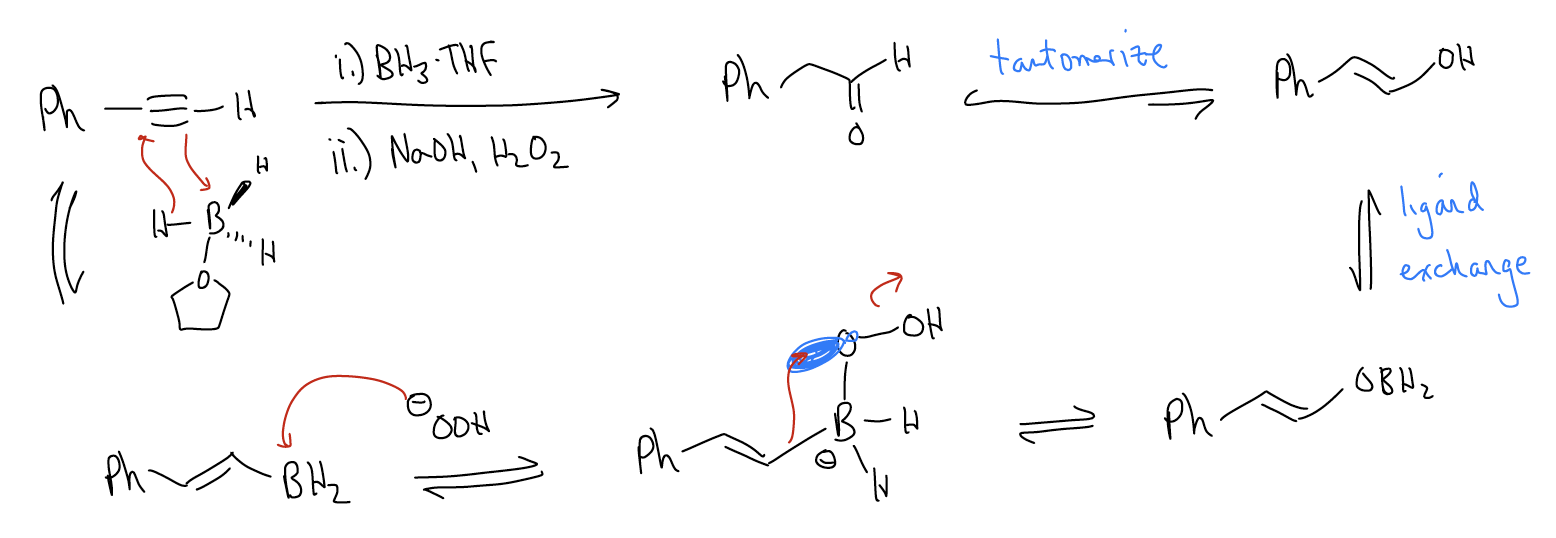
1. Hydroboration/oxidation – This will result in formation of a carbonyl on the less highly substituted carbon. In the case of an unsymmetrical internal alkyne, a ketone will result. In the case of a terminal alkyne, an aldehyde will result. Both products are formed by anti-Markovnikov [2+2] addition of \(σ_{B-H]}\) across the triple bond. Much like hydroboration/oxidation of alkenes, this is a syn-addition. Oxidation of the \(σ_{C-B]}\) bond with NaOH/H2O2 will generate an enol, which will tautomerize to the keto form.
C. [3+2] cycloadditions
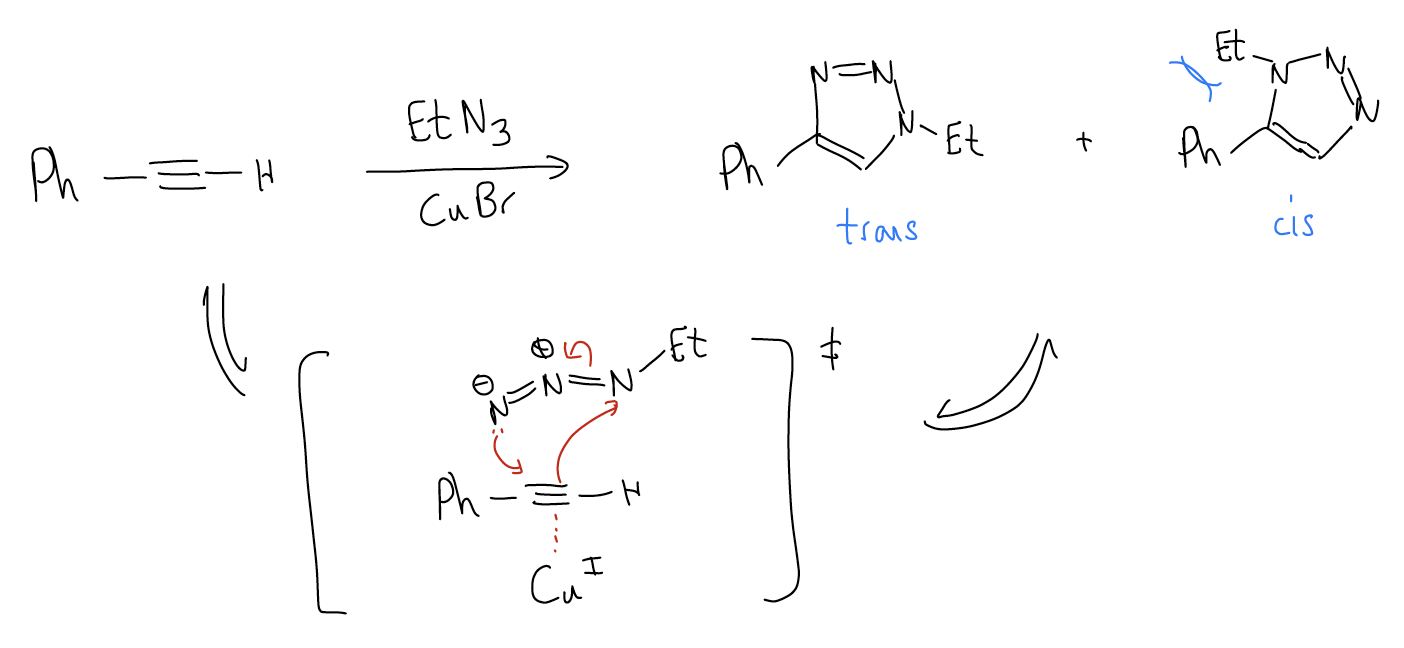
1. “Click” chemistry – This will result in formation of a triazole, where the “trans” regioisomer predominates. “Click” chemistry was popularized by K. Barry Sharpless as a convenient method of making heterocyclic rings. In this reaction, the alkyne participates in a [3+2] cycloaddition with organic azides. The reaction is catalyzed by Cu(I) salts. Formation of the trans-regioisomer predominates because the steric strain in the transition state is minimized when the largest groups are oriented away from one another.
Biomedical Spotlight
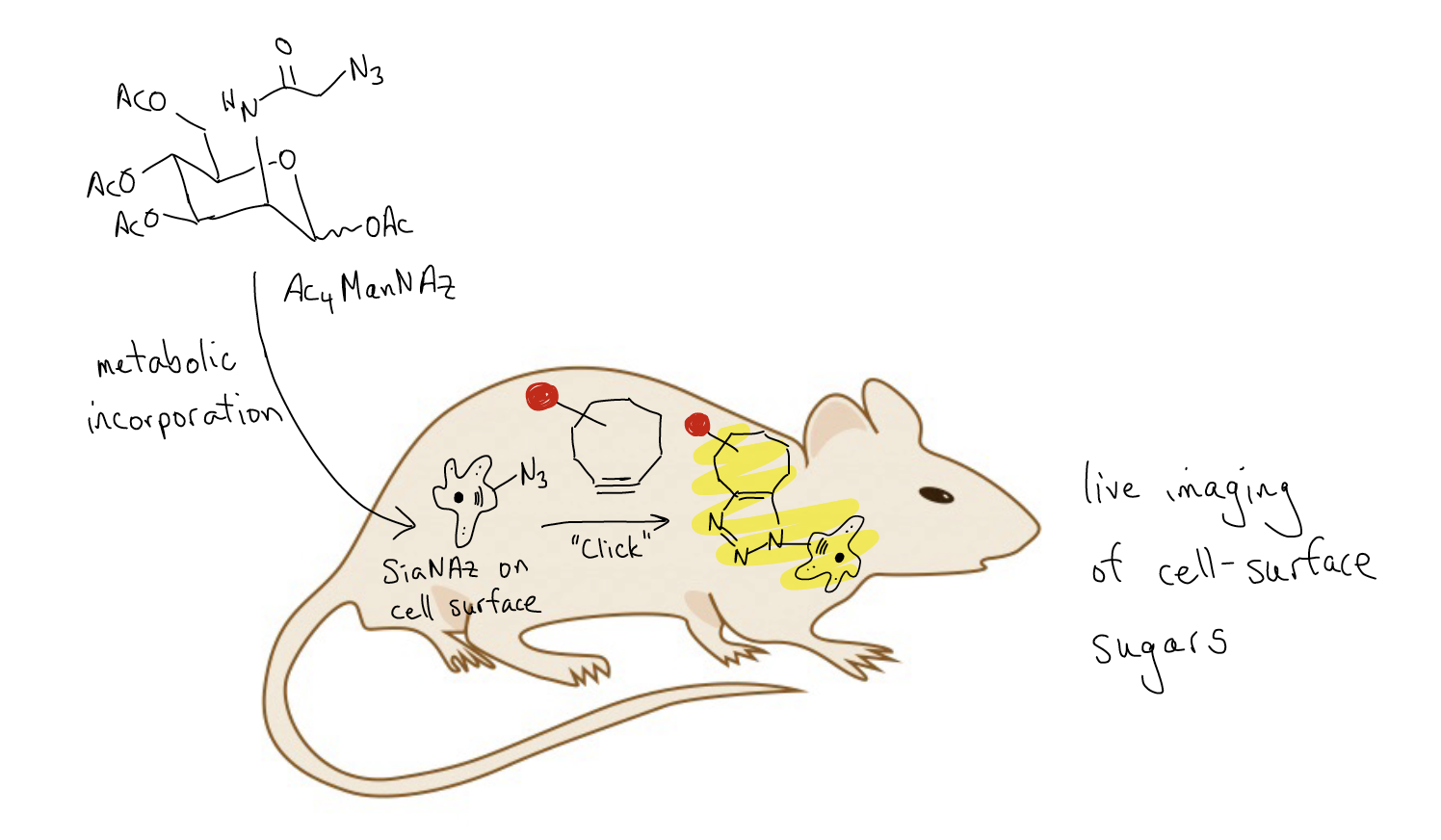
"Click" chemistry using azides and alkynes can be useful to create triazole heterocycles as a final target, but it can also be helpful for monitoring biological processes. The problem, however, is that copper is highly regulated in biological systems, and too much can be toxic to cells. Carolyn Bertozzi and coworkers published a study in 2010 (PNAS, 107 (5), 1821-1826) in which they took advantage of a cyclooctyne's inherent ring strain to facilitate triazole formation without the need for copper salts as additives. Bertozzi showed that a sugar that had been decorated with an azide group (Ac4ManNAz) could be metabolically incorporated into the carbohydrates that lie on the cell-surface (SiaNAz). By then adding an exogenous cyclooctyne that had been tagged with an imaging agent, a "click" [2+3] cycloaddition could occur to give a triazole that is appended to the cell surface. This allowed the group to visualize the appearance, location, and metabolism of carbohydrates on the surface of cells in real time.