11.2: Alkyne Reduction and Electrophilic Addition
- Page ID
- 321643
Alkynes react more readily under hydrogenation conditions (H2, Pd/C, etc.) than alkynes. The heat of hydrogenation of acetylene is more than twice that of ethylene. This reaction is more exothermic and should make sense because \(π\) bonds are weaker than \(σ\) bonds and alkynes have more of the former and fewer of the latter. There is a problem however with the hydrogenation of alkynes. You might think that the product of the hydrogenation of an alkyne is an alkene, but remember that alkenes also react under these conditions to give alkanes. Thus, reduction of alkynes will proceed all the way to the alkane.
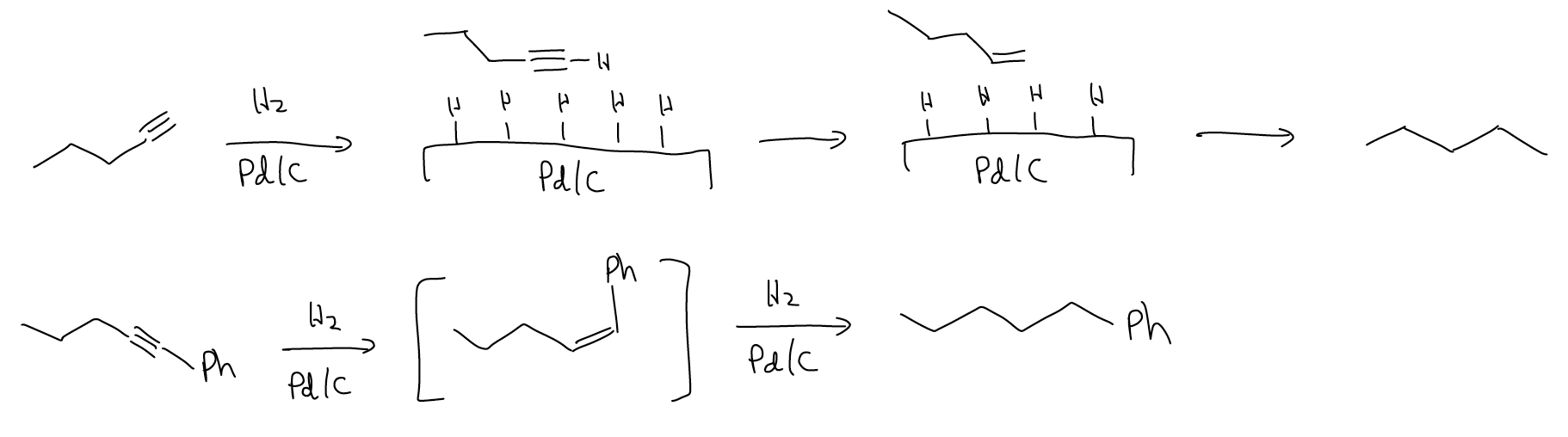
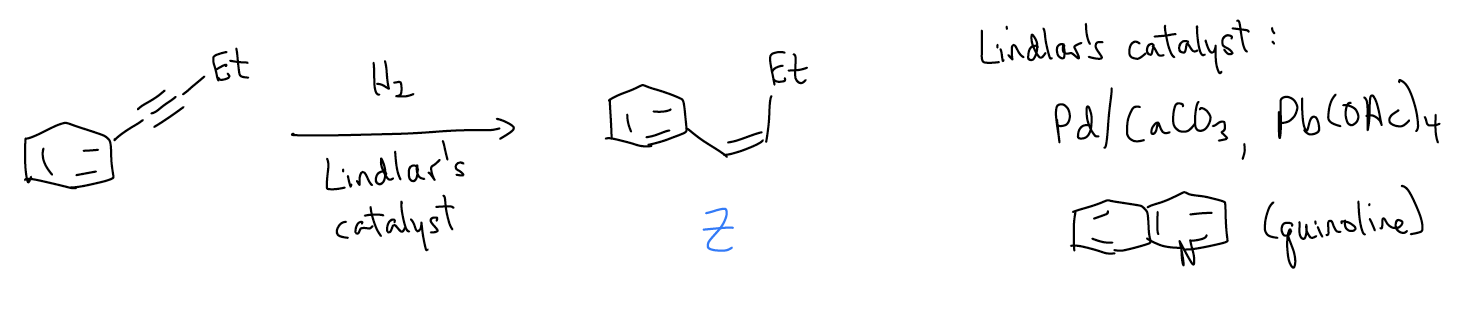
Is there a way to stop the hydrogenation halfway so that the alkene can be isolated? Of course, there is! Lindlar’s catalyst is a deactivated catalyst that is unreactive towards alkenes. It is a combination of Pd/CaCO3, Pb(OAc)4, and quinoline that partially poisons the palladium enough that alkenes will not become hydrogenated. Since the mechanism of this reaction is still a concerted, syn-addition of H2 across the triple bond, it is a good method of preparing cis-alkenes. This is in contract to the preparation of cis-alkenes via E2, where they will be a minor product due to steric strain in the transition state.
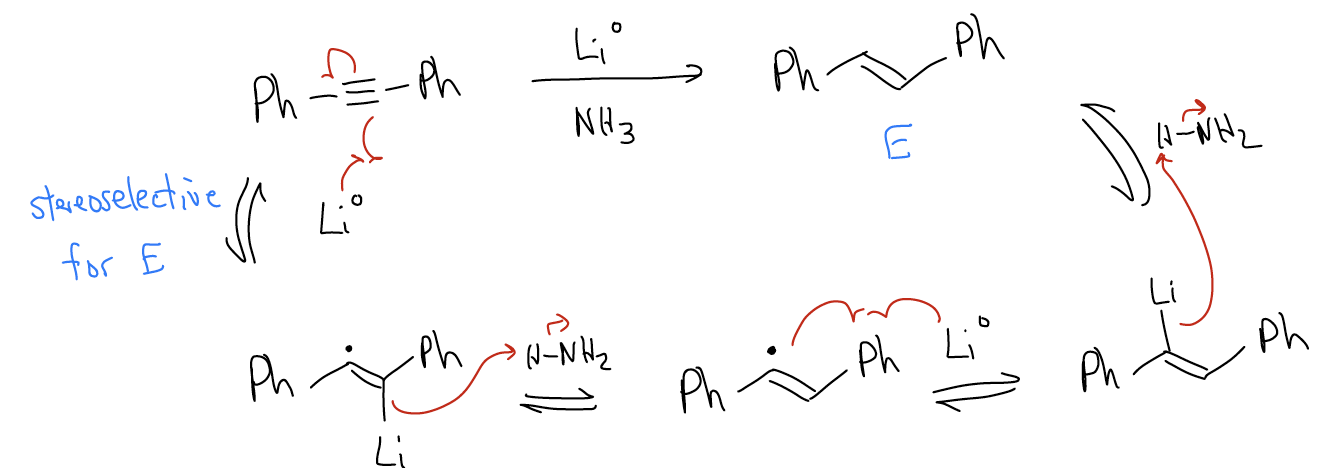
So, what about preparing E-alkenes from alkynes? This can be done by performing a dissolving metal reduction. The mechanism is quite different and involves one-electron transfer reactions, similar to the formation of the Grignard reagent. The initial one-electron transfer step is stereoselective, giving rise to the E-isomer. The reaction is a dissolving metal reduction since the metal actually dissolves as the reaction progresses.
Let’s now consider all of the other reactions of alkenes (where \(π_{C-C}\) is the HOMO) and how they might be applied to alkynes.
Electrophilic addition
a. Hydrohalogenation - This will result in formation of a vinyl halide that is a Markovnikov addition of H-X across the triple bond. The terminal alkynyl carbon has the largest coefficient in the HOMO, so it is most nucleophilic. The rate of this reaction is third order, which suggests a different mechanism is operative. There is no formation of a vinyl carbocation, but rather two molecules of H-X are involved in the transition state. This mechanism is called an addition-electrophilic-termolecular (AdE3), and is still not quite well understood.
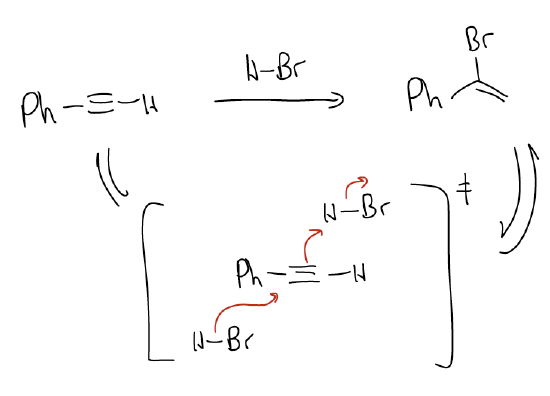
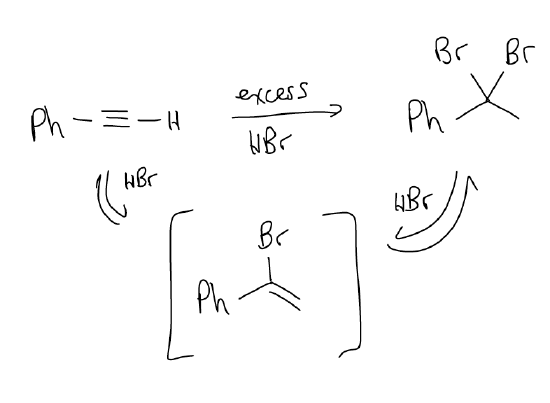
Vinyl halides are notoriously reactive, and in excess H-X, will react again via electrophilic addition to give a geminal dihalide, also via Markovnikov regiochemistry.
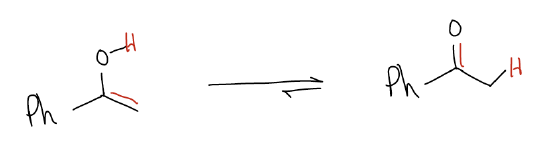
b. Hydration – This will result in formation of a ketone, formed by addition of water across the triple bond in a Markovnikov fashion. Normally, this proceeds via an AdE3 mechanism where water attacks the more highly substituted carbon while simultaneously protonating the less highly substituted carbon. Mercury(II) salts are commonly used in this reaction. The net result of the initial AdE3 mechanism is an enol, which rapidly tautomerizes to the keto form
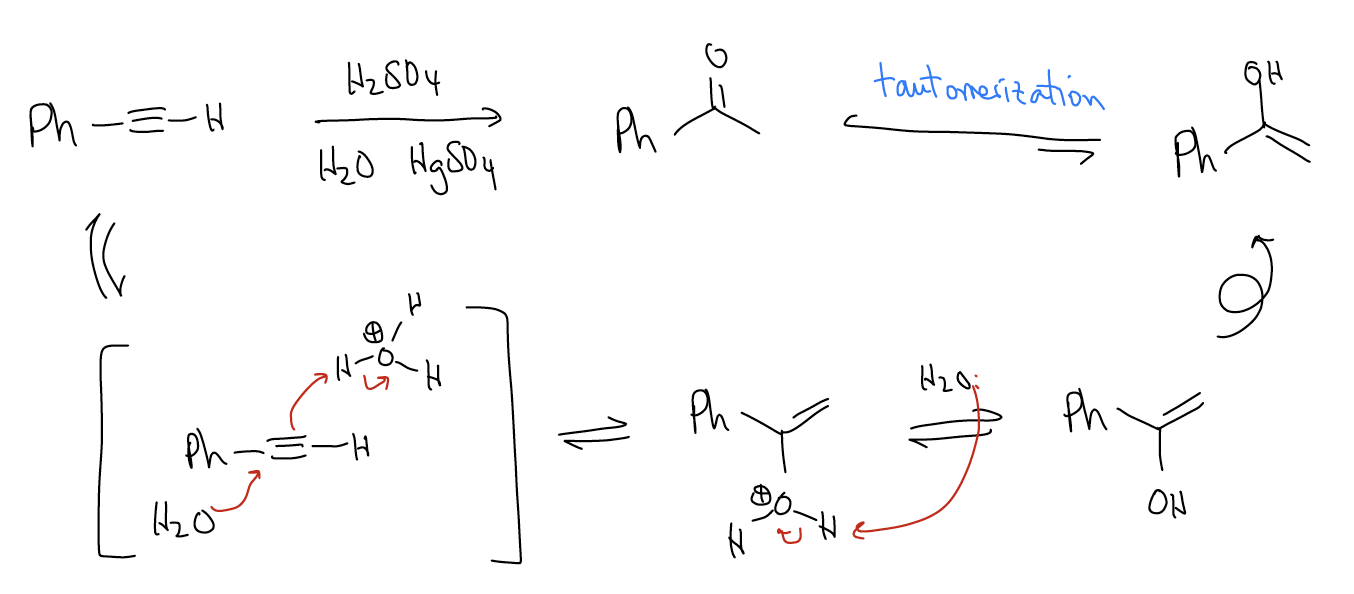
Tautomerization is a consequence of resonance and occurs rapidly at room temperature. The keto form is more stable than the enol form, so hydration of alkynes is a good method of preparing ketones. In a retrosynthetic sense, be mindful that this is difficult to see because it is not readily apparent from the structure of the keto form that can come from an alkyne.