8.3: The Basics of Determining Mechanism
- Page ID
- 321474
By now, you’ve been exposed to only a few chemical reactions whereby a simple starting material is converted into a more complex product. Take, for example, the electrophilic addition reaction. In this reaction, an alkene is converted into the more highly substituted alcohol (or alkyl halide) by reaction with water and an acid like H2SO4 or H3PO4 (or a mineral acid like HCl, HBr, and HI). The mechanism is protonation of the alkene (\(π_{C-C}\) = HOMO) at the less highly substituted carbon atom of the alkene to form a stable carbocation, which then gets trapped by a nucleophile (followed by deprotonation, if applicable).
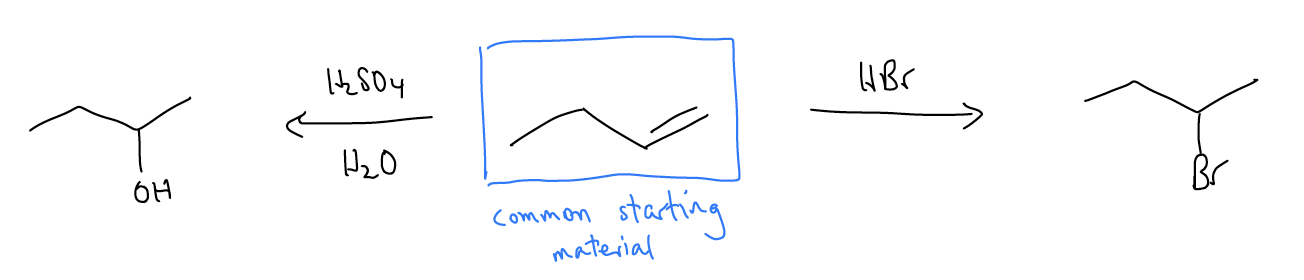
As you will come to appreciate soon, there is often more than one way to make a particular molecule. For example, there is another method of making an alkyl halide that does not use an alkene as the starting material. The reaction between an alcohol and an inorganic acid (hydrogen halide) results in a chemical reaction that generates an alkyl halide and water. Each of the reactants (alcohol and HX) has an impact on the reactivity (how well the reaction works).
So, how does this reaction work? We are making and breaking bonds in the process, so how could we draw a set of curly arrows that would get us from starting material to product? We have come across this type of thinking before. It involves a description of the step-by-step pathway from starting material to product. This is known as the mechanism. Mechanisms are based on experimental evidence and can never be proven (however, mechanisms CAN be disproven). One may be able to think of a variety of ways of constructing curly arrows in a reaction mechanism, but understanding whether they are reasonable is more important. Say, for example, you wanted to get from New York to Los Angeles within 24 hours. There are a variety of ways you can get there (car, train, plane), a variety of routes to take, etc. But if you told me that you arrived by boat, I’d think you were crazy. The same thing happens with organic reaction mechanisms – we must always be checking ourselves to see if everything makes sense.
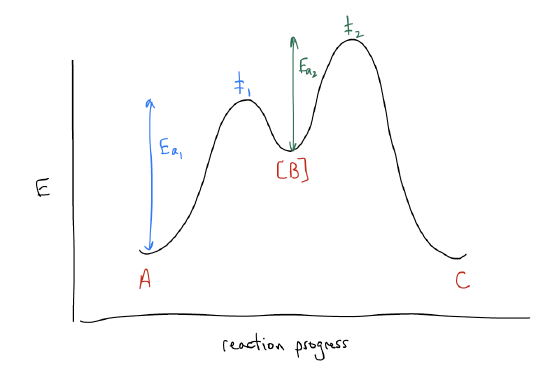
The reaction we just described is known as an SN1 reaction, which stands for nucleophilic substitution, 1st order. What this means is that the rate of the reaction is dependent only on the presence of one molecule in the rate determining step, hence 1st order. The rate determining step, or slowest step, involves only one molecule, and the energy required to get this one molecule to make or break bonds is the largest (compared to the other individual bond forming/breaking events). Potential energy diagrams are useful in thinking about reaction mechanisms. Consider the following generic reaction: A → [B] → C, where A is starting material, B is an intermediate, and C is a product. A point of maximum potential energy is known as a transition state. The difference in energy between an energy well and a transition state is called the activation energy. If the energy level of the products is lower than that of the reactants, we say that the reaction is exothermic (vice versa for endothermic). The intermediate B is very high in energy and normally cannot be detected because it is so transient. Transition states are not stable structures and cannot be detected because they involve both bond-making and bond-breaking events. According to the Hammond postulate, they resemble the energy well that is closest in energy. So, for TS #1 and TS #2, they look mostly like intermediate B. The rate of this reaction depends on the height of the highest transition state and everything that comes before. So, the rate will depend on the structure and concentration of B, in this case.