3.3: Conformations of Substituted Hydrocarbons
- Page ID
- 319716
B. Conformations of substituted hydrocarbons
We have said that for straight chain or branched hydrocarbons, the most stable conformation will be staggered and preferably antiperiplanar (if not, at least minimizing gauche interactions). This is due primarily to three principles: torsional strain, \(σ\)-donation, and steric strain. It turns out that steric factors are not as strong as electron factors (torsional and \(σ\)-donation). Here’s why:
Consider the molecule ethylene glycol, a common component of antifreeze. Draw the three most stable conformations down the barrel of the C-C bond:
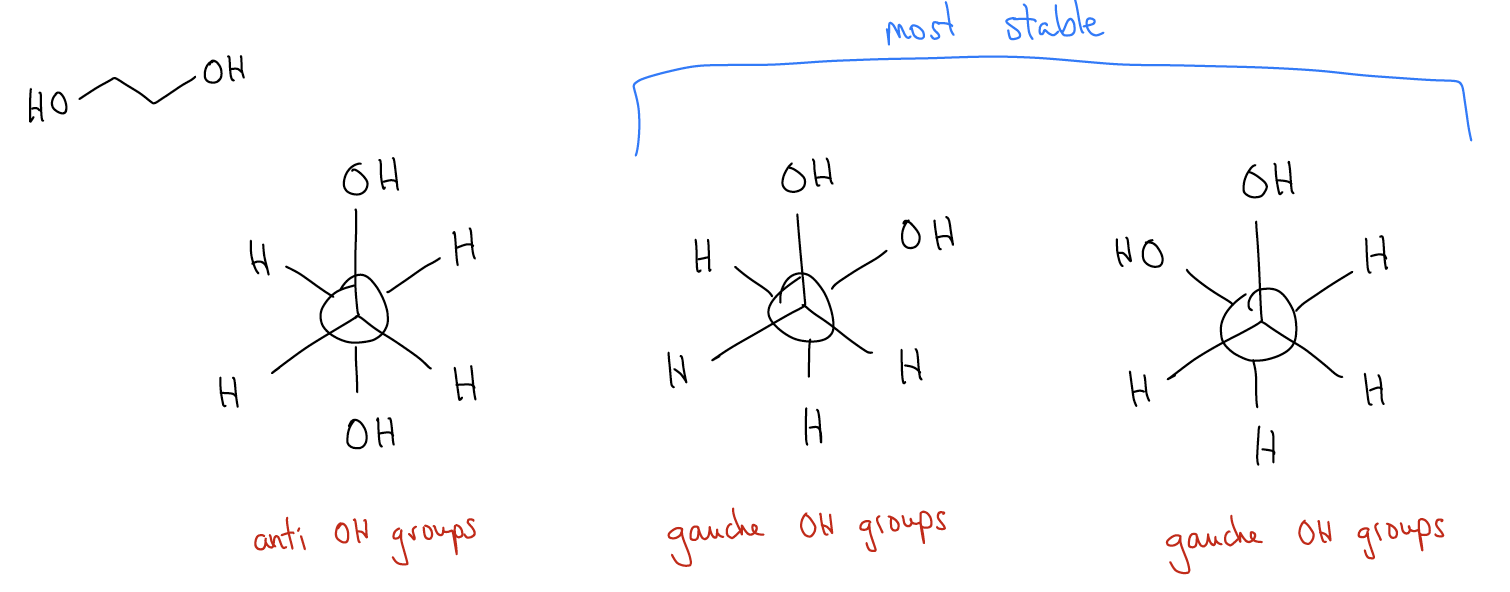
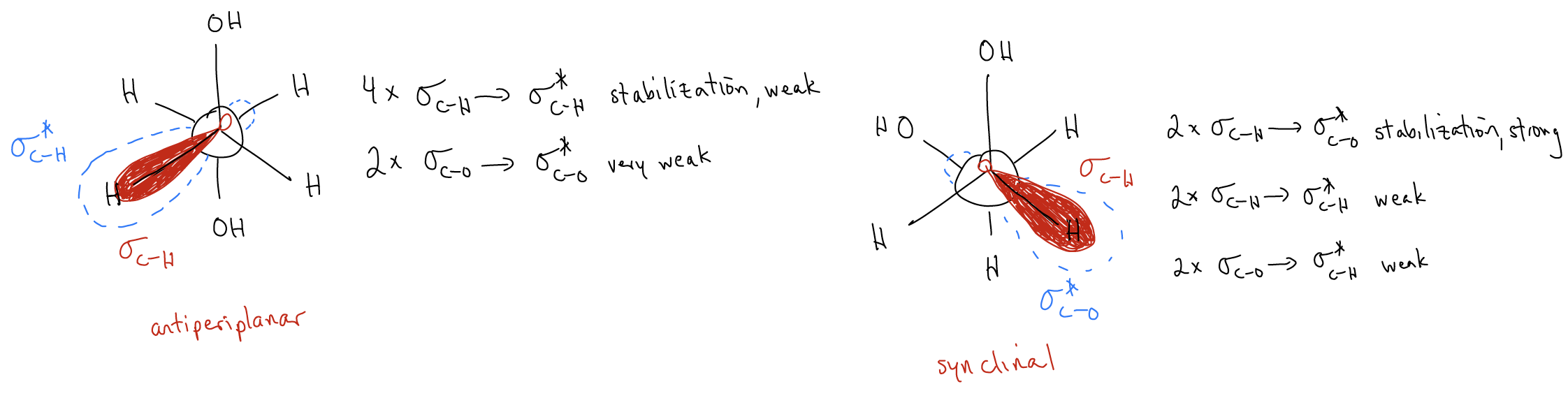
Which of these is more stable? It is actually the gauche conformations! While in the antiperiplanar conformation there is minimal steric clash, the delocalization of electrons between \(σ_{C-H}\) and \(σ^{*}_{C-H}\) is weak. However, in the two gauche conformations, the delocalization of electrons is between \(σ_{C-H}\) and a much lower energy \(σ^{*}_{C-O}\). There are two such interactions and since \(σ^{*}_{C-O}\) is lower in energy due to electronegativity, this has a powerful stabilizing effect for the electrons in \(σ_{C-H}\) (they can delocalize into a much lower energy orbital). This cannot happen in the antiperiplanar conformation.
Here’s another example. Draw the three most stable conformations for 1,2-difluoroethane and decide which is the most stable.
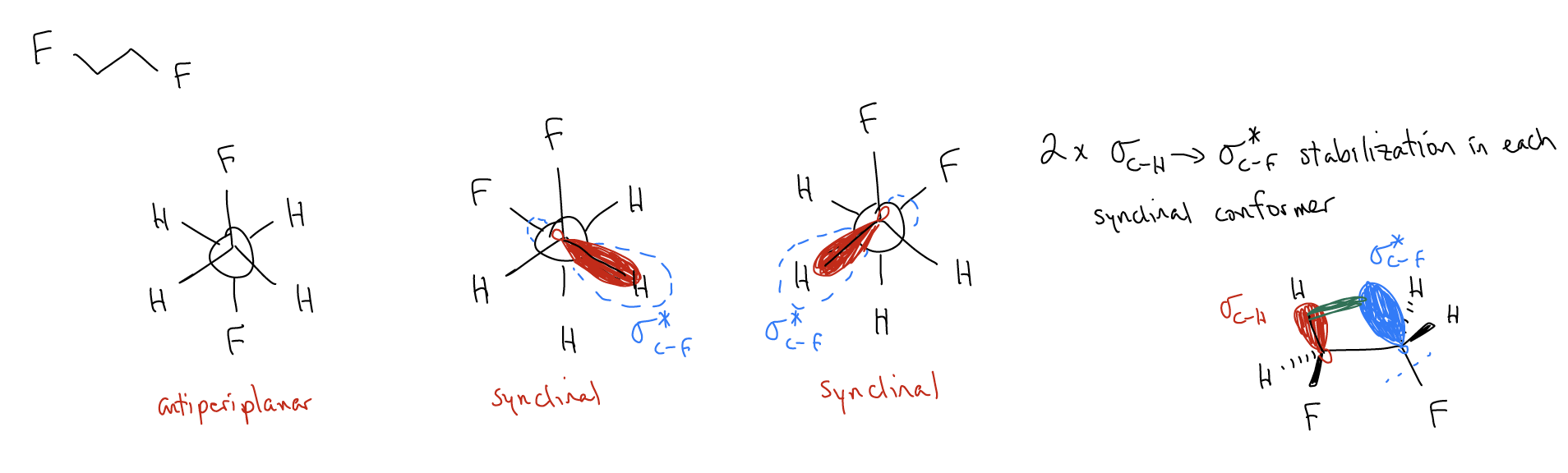
Again, there is better \(σ\)-donation between \(σ_{C-H}\) and \(σ^{*}_{C-F}\). Overlap of these orbitals creates a new molecular orbital with even lower energy than before! This is called the gauche effect.
One more example, this time involving lone pairs. Draw the most stable conformations for hydrogen peroxide:
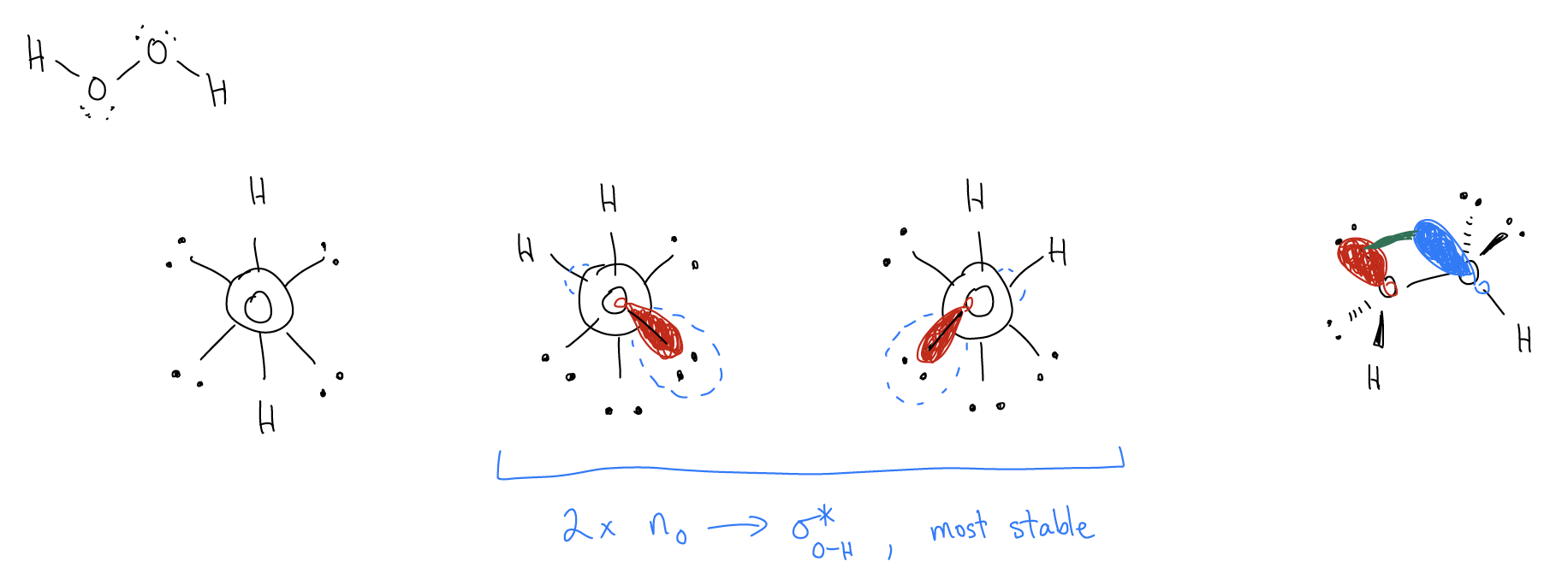
This time, the two gauche conformations have nonbonding electrons on oxygen (nO) that can delocalize into a low lying antibonding orbital (\(σ^{*}_{O-H}\)). Nonbonding electrons are always higher in energy than \(σ\) electrons, so the maximum stabilization occurs in the gauche conformation again.

