2.2: Molecular Orbital Theory
- Page ID
- 319625
Molecular Orbital Theory – 3rd generation model in response to limitations of valence bond model that help describe reactivity. Just as electrons occupy atomic orbitals, electrons in molecules (namely, in chemical bonds) occupy molecular orbitals. Molecular orbitals are described as combinations of atomic orbitals, known as the linear combination of atomic orbitals:
Rule #1: for every n atomic orbitals, one will create n molecular orbitals. So, if you start with a 1s orbitals of H and another 1s orbital of H and combine them to form a chemical bond, you will generation two molecular orbitals overall. We call these the bonding (\(σ\)) and antibonding (\(σ^{*}\)) orbitals, derived from in-phase and out-of-phase overlap of the wavefunctions, respectively. The \(σ\) bond is lower in energy than the 1s atomic orbital and the \(σ^{*}\) is higher in energy than the 1s atomic orbital.
Rule #2: these two new molecular orbitals will have different energies. The lowest molecular orbital (\(σ\)) will have zero nodal planes and each successive orbital thereafter will have an additional nodal plane.
Rule #3: the coefficients, C1 and C2, represent the contribution of each atomic orbital to the overall wavefunction:
- bonding orbital: \(σ\) = C1\(Ψ\)1 + C2\(Ψ\)2
- antibonding orbital: \(σ^{*}\) = C1\(Ψ\)1 - C2\(Ψ\)2
In symmetrical molecules, C1 and C2 will be the same, but this will rarely occur in more complicated systems. The size of the coefficient will tell you which side of the bond has the bigger lobe for that orbital. In addition, the squares of the coefficients will always equal one (that is, (C1)2 + (C2)2 = 1) and both wavefunctions will contribute one net orbital (that is, bonding(C1)2 + antibonding(C1)2 = 1).
Let’s take the example of H2. Just as in the valence bond model, when two atomic orbitals overlap in-phase, we get net bonding (\(σ\)), but when they overlap out-of-phase, we get destructive interference and a nodal plane results (\(σ^{*}\)).
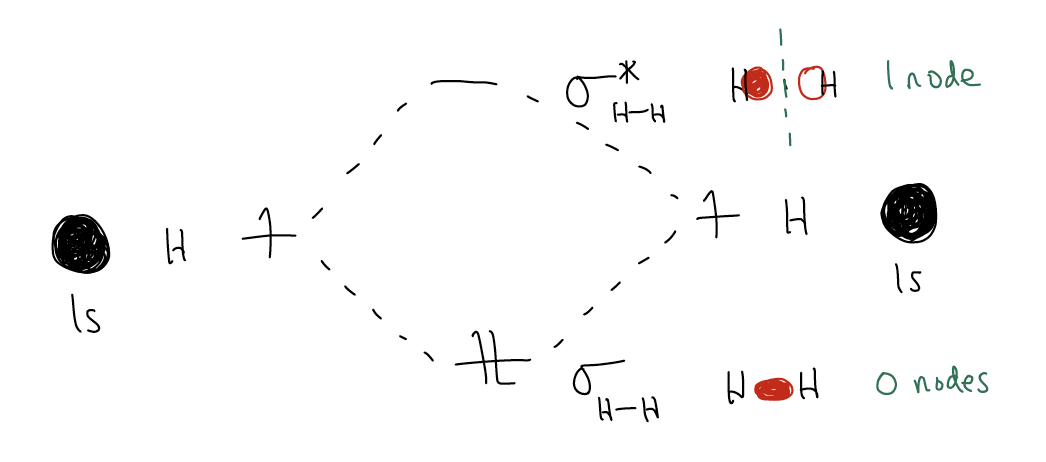
Given the molecular orbital diagram above, can you draw one for H2+?
When two atomic orbitals combine, the bonding orbital is lower in energy (s → \(σ\)) and you fill in the electrons from lowest energy to highest energy. What this tells us, then, is that in H2, there is net bonding between the H atoms. Convince yourself that the bond order is one. Now, say we were to fill the \(σ^{*}\) orbital with an electron. By doing so, the bond order will decrease to ½ and the \(σ\) bond will weaken. Add two electrons, and the \(σ\) bond will break completely (bond order = 0). This is why molecular orbital theory is so important – it gives us an entry into reactivity. It allows us to break bonds!

