16.2: Ionizers
- Page ID
- 272077
The function of an ionizer is to convert the particles in a sample into gas phase ions. In addition to the types of samples each ionizer can handle, the big distinction is whether the ionization process is hard or soft. Hard ionizers produce ions with a great deal of excess internal energy leading to fragmentation. Hard ionizers less likely to produce the molecular ion, M+. Soft ionizers produce considerably less fragment ions and are very likely to produce the molecular ion or a quasi molecular ion. A quasimolecular ion is an ion formed by the association of the molecule, M and a known charged species, i.e. MH+ or MNa+.
Figure \(\PageIndex{1}\) gives an example of hard ionization versus soft ionization for the nerve gas agent VX.
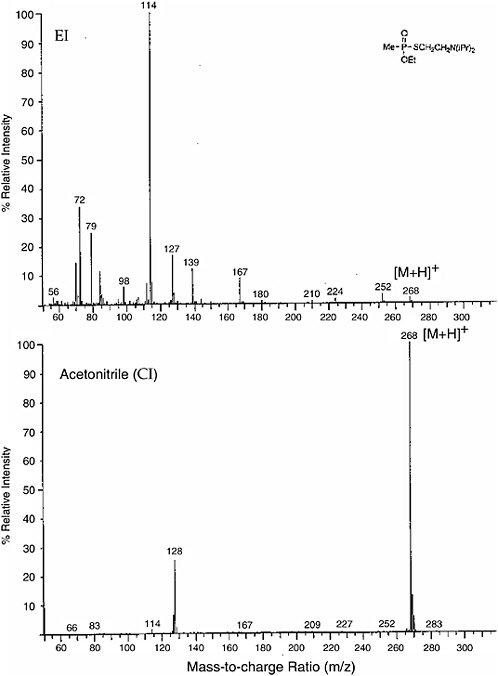
Figure \(\PageIndex{1}\): The mass spectra obtained for agent VX using electron impact ionization (hard) and chemical ionization (soft). Image from www.nap.edu but presented in https://socratic.org/questions/when-...bardment-ei-ms
The intensity of the peak for the quasi molecular ion of agent VX (VX-H+) is much more intense with CI and there is only one significant fragment ion (daughter ion) peak at (m/ze) = 128 coresponding to the loss of the neutral fragment associated with rupture of the S - C bond. electron impact ionization produced a spectrum with many more fragment ion peaks.
The Electron Impact Ionizer
The electron impact ionizer (EI) is a hard ionization method. As shown below in Figure \(\PageIndex{2}\), electrons emitted thermionic emission from a hot filament are accelerated across the ionizer. Energetic collisions between the accelerated electrons and gas phase sample species, M. The collision results in the loss of one (or more) electrons; M + e- → M+ + 2e-.
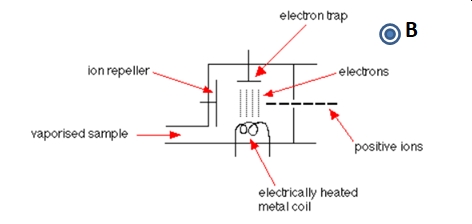
Figure \(\PageIndex{2}\): A simple sketch of an electron impact ionizer. The magnetic field is increases the path of the electrons across the ionizer leading to more ionizing collisions.
A generic organic molecule has an ionization energy on the order of 5 eV (500 kJ/mole). So electrons with kinetic energies > 5 eV will produce ions with the degree of fragmentation increasing as the kinetic energy of the electrons increases. However at low electron kinetic energies all molecules are not ionized equally well. A electron kinetic energy of 70 eV is commonly employed for EI ionization and mass spectrometry libraries are based on this value
Chemical Ionization
Chemical ionization is a soft ionization method based on ion-molecule reactions occurring in an electron impact ionizer. As shown in Figure \(\PageIndex{3}\) reagent gas molecules are introduced at a concentration about 100X greater than the sample particles. The reagent molecules, R, are ionized by the energetic electrons to for a reagent ion, R+. The reagent ions react with the sample molecules, S, to produce the ions, S+, to be sent to the mass analyzer.
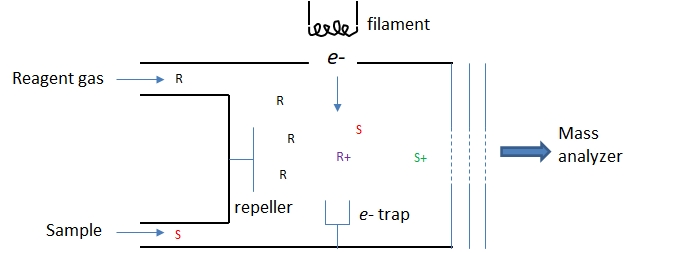
Figure \(\PageIndex{3}\): A simple sketch of a chemical ionization source.
Tehe reagent ions employed in chemical ionization are commonly based on methane (CH5+, C2H5+), ammonia (NH4+), or the nobel gases. The types of ion-molecule reactions used to produce ions in a chemical ionization source are shown in the table below. All these reaction are exothermic, however, the excess energy of the ion produced is much less than that produced by EI ionization.
Ion-Molecule Reactions used to make analyte ions from MH
| CH5+ + MH → MH2+ + CH4 | \({\Delta}\)H < 0 | proton transfer |
| C2H5+ + MH → MH2+ + C2H4 | \({\Delta}\)H < 0 | proton transfer |
| NH4+ + MH → MH2+ + NH3 | \({\Delta}\)H < 0 | proton transfer |
| C2H5+ + MH → M+ + C2H6 | \({\Delta}\)H < 0 | hydride transfer |
| Ar+ + MH → MH+ + Ar | \({\Delta}\)H < 0 | charge transfer |
The \({\Delta}\)H for proton transfer reactions range from - 0.01 to - 0.5 eV (-1 to -50 kJ/mole) while the \({\Delta}\)H for charge transfer reactions - 0.01 to - 0.2 eV (-1 to -20 kJ/mole). The excess energy imparted to teh ion produced and consequently the extent of ion fragmentation can be varied by the choice of reagent ions. For example, in a charge transfer ionization between a He ion (ionization energy 24.5 eV) and a sample molecule with an ionization energy of 5 eV will leave the ion produced with approximately 19.5 eV of excess energy. Charge transfer ionization of the same molecule with argon ions (ionization energy 15.7 eV) or krypton ions (ionization energy 14 eV) will leave the ion with an excess energy of approximately 10.7 eV and 9 eV respectively
Fast Atom Bombardment (FAB)
Fast atom bombardment (FAB) is a soft ionization technique that produces positive ions, negative ions, and quasi molecular ions in an energetic collision between a fast moving atom and sample molecule contained in a viscous matrix. As shown in Figure \(\PageIndex{4}\), argon ions produced via electron impact are accelerated to very large kinetic energies (8 - 35 keV)and directed towards the target copper probe tip. These fast moving ions are directed into a gas chamber containing slow moving, thermal energy, argon atoms. Electrons transferred in a net energy neutral reaction from the atoms to some of the fast moving argon ions produce fast moving argon atoms. Only fast moving atoms strike the target as any remaing fast moving ions are directed off the target path using electrostatic deflector plates.
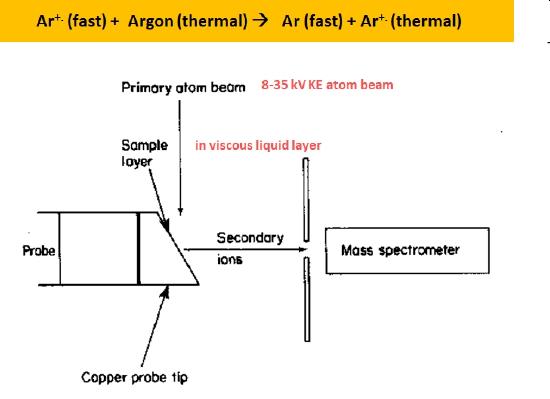
Figure \(\PageIndex{4}\): A schematic illustration of a fast atom bombardment (FAB) ionization source.
While both positive ions and negative secondary ions are produced in collision between the fast argon atoms and the sample molecules in the matrix only one type of ion is sent to the mass analyzer. One can either analyze positive ions or negative ions at a time, not both. It should be noted that multiply chaged ions are often produced in a FAB source.
Matrix Assisted Laser Desorption Ionization (MALDI)
Pulsed lasers such as the nitrogen laser (337 nm) or a pulsed Nd:Yag laser (1064 nm, 532 nm or 355 nm) are capable of delivering millijoules of energy in a 5 - 10 nsec pulse of light. Consequently and as shown in Figure \(\PageIndex{5}\) a matrix assisted laser desorption ionization source look very much like a FAB ionization source with the fast atom beam replaced by the light beam from a pulsed laser.
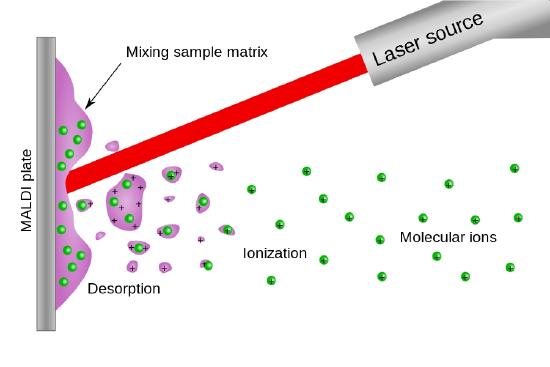
Figure (\PageIndex{5}\): An schmatic illustration of a MALDI source. Image from the Wikimedia Commons.
As with the FAB ionization source, MALDI produces both positive ions and negative secondary ions, quasi molecular ions and multiply charged ions. There are many receipies for the matrix in a MALDI source, some which add species that absorb at the wavelength of the pulsed laser.
Because of the pulsed nature of the MALDI source this type of ionizer is often coupled with a Time-of-flight (TOF) mass analyzer.
Thermospray
The thermospray ion source is one of the first sources designed specifically to couple a HPLC or syringe pumped capillary to a mass spectrometer. As shown in Figure (\PageIndex{6}\) a sample solution is pumped into a heated stainless-steel capillary, rapid evaporation of solvent from the liquid surface occurs, resulting in an ultrasonic spray of vapor and charged droplets. Disintegration of the charged droplets occurs repetitively due to continuous evaporation of solvent and the Coulombic repulsion between like charges. The process eventually causes ions,as well as neutral molecules, to be released from the surface of the microdroplets. The ions are extracted and accelerated toward the analyzer by an electrostatic system voltage.

Figure (\PageIndex{6}\): A schematic illustration of a thermospray ionization source . In this figure A 100 W cartridge heaters, B copper block, C stainless steel capillary 0.15 mm id, D copper tube, E ion lenses, F quadrupole mass analyzer. Ref.: C. R. Blakley, M. L. Vestal, Anal. Chem. 55, 750 (1983).
Thermospray is a soft ionization technique producing both positive ions and negative secondary ions, quasi molecular ions and multiply charged ions. When applied to studies of proteins, biological macromolecules with multiple acid or base groups, the distribution of charges on the ions of the same species can be greatly influenced by the pH of the buffer solution.
The thermospray source is more of historical importance as the electrospray source and the atmospheric pressure interface are much more commonly found in today's instruments.
Atmospheric Pressure Ionization (API)
In addition to the electrospray ion source described in a later subsection in this chapter, the atmospheric pressure ionization interface is widely used to couple HPLC's and syringe pumped capillaries to mass spectrometers. As shown in Figure (\PageIndex{7}\) a small flow solution is passed through a heated zone as in a thermospray source and nebullized. The resulting aerosol mist is passed by a sharp needle held at 2 - 4 kV producing a corona discharge. The resulting solvated ions are allowed to lose solvent molecules by evaporation and a sample of the ion cloud is passed into the mass analyzer.
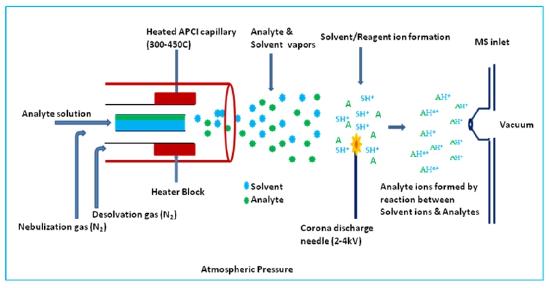
Figure (\PageIndex{7}\): A scehmatic illustration of a atmospheric pressure interface. Image from https://www.chem.pitt.edu/facilities...y-introduction.
As with the thermospray and electrospray API is a soft ionization technique producing both positive ions and negative secondary ions, quasi molecular ions and multiply charged ions. When applied to studies of proteins, biological macromolecules with multiple acid or base groups, the distribution of charges on the ions of the same species can be greatly influenced by the pH of the buffer solution.

