14.3: Chiral Chromatography
- Page ID
- 272006
The separation of enantiomers of chiral compounds by chromatographic methods and related techniques is one of the most important tasks in modern analytical chemistry, especially in the analysis of compounds of biological and pharmaceutical interest. While chiral separations can be accomplished using gas chromatography they are more often accomplished with liquid chromatography and capillary electrophoresis due to the focus on molecules of importance to the pharmeceutical industry.
When the enantiomers of a drug are administered into a chirally selective living system, these enantiomers often exhibit differences in bioavailability, distribution, metabolic and excretion behavior, and action. One of the enantiomers is often the more active stereoisomer for a given action (eutomer), while the other, less active one (distomer) may either contribute side-effects, display toxicity or act as an antagonist. The differences in biological properties of enantiomers arise from the differences in protein transport and binding, the kinetics of their metabolism and their stability in the environment. A tragic case in point is the racemic drug n-Thalidomide which was marketed in the the United Kingdom late 1950's as a sedative and with tragic effect. Exclusively the R-(+) enantiomer possesses therapeutic activity while the S-(+) enantiomer is teratogenic.
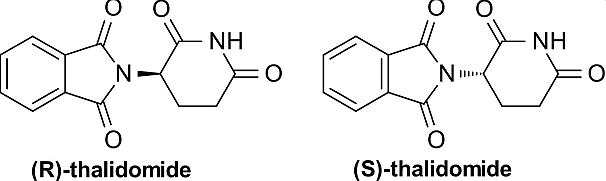
As a result, the pharmaceutical industry has raised its emphasis on the generation of enantiomerically pure compounds in the search for safer and more effective drugs. Single enantiomers can be obtained via (a) the selective synthesis of one enantiomer or (b) the separation of racemic mixtures Stereoselective syntheses are rarely selected for largescale separations, particularly at the early stages of development of new drugs in the pharmaceutical industry because they are both expensive and time-consuming. Yet, These methods yield the enantiomerically pure substances and are generally the most advantageous and are a major focus of modern synthetic chemistry. The enantiomers of a racemic mixture can be separated indirectly when diastereomer pairs are formed covalently; their separation can be achieved by taking advantage of their different chemical or physical properties on crystallization, nonstereoselective chromatography or distillation. Alternatively, direct processes are based on the formation of noncovalent diastereomeric pairs of molecules. The direct resolution of enantiomers can be achieved by interaction of a racemic mixture with a chiral selector, either a part of a chiral stationary phase (CSP) or as a chiral mobile phase additive (CMA).
Indirect chromatographic methods
The application of chiral derivatizing agents (CDAs) to produce diastereomer pairs with appropriate separation and detection possibilities was the first method widely used for the enantioseparation of optically active molecules in liquid chromatography. Labeling with CDAs is carried out by reaction with a functional group in the analyte, e.g. amine, carboxy, carbonyl, hydroxy or thiol.
Among various functional groups, the tagging reactions of primary and secondary amines are unquestionably of major significance. The derivatization reactions for chiral amines are mainly based on the formation of amides, carbamates, ureas and thioureas. Only the most important members of the CDA types are shown in Figure \(\PageIndex{1}\).
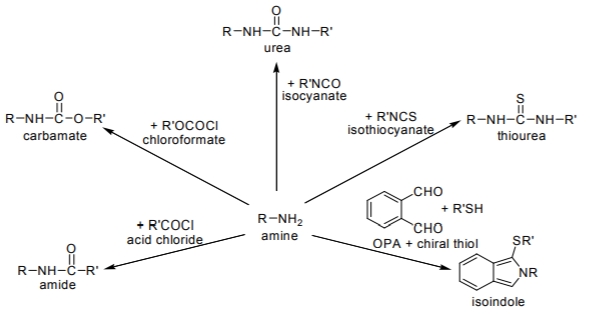
Figure \(\PageIndex{1}\): Some examples of chiral derivatization reactions for amino groups (both R and R’ contain a chiral center) leading to the formation of diastereomer pairs for each solute enantiomer.
Advantages of indirect methods for chiral separations include: 1. Good chromatographic properties of derivatives 2. Elution sequence predictable 3. Good chromophoric or fluorophoric properties of the reagent (enhanced sensitivity can be achieved) 4. Low cost of achiral columns 5. Method development is simple and 6. Selectivity can be increased (better separation is often achieved than with a direct method). Disadvantages include: 1. The purity of the CDA is critical and 2. The excess of reagent and side-products may interfere with the separation.
Direct Chromatographic Methods
Chiral Mobile Phase Additives (CMAs)
Enantiomers can be resolved on conventional achiral stationary phases by adding an appropriate chiral selector to the mobile phase. These additives can be cyclodextrins, chiral crown ethers, chiral counter-ions or chiral ligands which are capable of forming ternary complexes with the solute enantiomers in the presence of a transition metal ion.
Chiral Stationary Phases
The 1980s proved to be a major turning-point in the field of enantiomer separation in HPLC. A tremendous number of new and improved CSPs were introduced and accompanied by a corresponding increase in the number of publications in this area. CSPs can be grouped in several ways. Depending on their separation principles, the main classes are as follows: Pirkle CSPs, helical polymers, mainly cellulose and amylose, cavity phases, macrocyclic antibiotic phases and protein- and ligand-exchange phases.
Pirkle Phases
The first commercial chiral stationary phase was developed in W. Pirkle's laboratory at the University of Illinois in the 19080's. This chiral stationary phase was based on immobilized (R)-2,2,2-trifluoro-1-(9-anthryl)ethanol (previously used in magnetic resonance studies) on a silica support. This CSP was shown to be useful for the separation of enantiomers of several π-acidic racemates, e.g. 3,5-dinitrobenzoyl (DNB) derivatives of amines, amino acids, amino alcohols, etc. pi-Basic phases such as 1-aryl-1-aminoalkanes, N-arylamino esters, phthalides, phospine oxides, etc. contain a pi-electrondonor group and are expected to interact and resolve compounds bearing a π-electronacceptor group. Typically, DNB and 3,5-dinitrophenylurea derivatives of amino acids and amines were resolved on these CSPs. Among the DNB derivatives, phenylglycine exhibited large separation factors. This observation led to the investigation of DNB phenylglycine as a pi-acid-based CSP. Finally, the Pirkle laboraotry designed CSPs containing both pi-acidic and pi-basic sites [63]. These phases were expected to allow the efficient resolution of compounds containing appropriately located pi-acidic and pi-basic moieties. Pirkle and his coworkers commercialized many of their CSP sold through Regis Technologies (registech.com) where CSPs such as the very versatile Welk-O 1, shown in Figure \(\PageIndex{2}\) are named after the co-worker that developed the CSP.
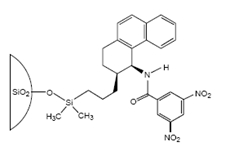
Figure \(\PageIndex{2}\): The Whelk-O 1 chrial stationary phase sold by Regis Technolgies. Image from registech.com.
CSPs based on polysaccharides
Polysaccharides (especially cellulose) are naturally-occurring polymers; their derivatives were found to exhibit the ability of chiral recognition as CSPs. Cellulose is a crystalline polymer composed of linear poly-β-D-1,4-glucoside residues which form a helical structure. Although crystalline cellulose displays chiral recognition, it does not yield practical CSPs. The reason for this is the poor resolution, and broad peaks are obtained due to slow mass transfer and slow diffusion through the polymer network. The highly polar hydroxy groups of cellulose often lead to nonstereoselective binding with the enantiomers of the analyte. Additionally, cellulose is unable to withstand normal HPLC pressures.
Derivatization of cellulose brings about practically useful CSPs which can separate a wide range of racemic compounds with high selectivities. Among the derivatives, cellulose tris-4-methylbenzoate and tris-3,5-dimethylphenyl carbamate, shown in Figure \(\PageIndex{3}\)vatives containing both an electron-donating and an electron-withdrawing group on the phenyl moiety were prepared to perfect their chiral recognition abilities.
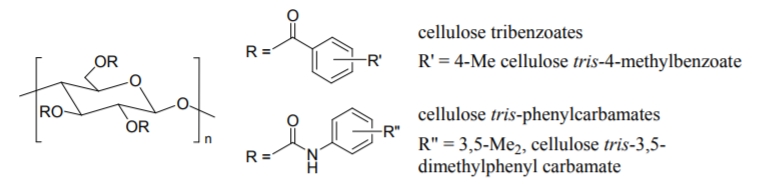
Figure \(\PageIndex{3}\): Two examples of derrivatized polysaccaride CSPs based on celluose.
Cavity phases
Chiral separations based on inclusion are achieved through a mechanism by which the guest molecule is accepted into the cavity in a host molecule. The exterior of the host molecule generally possesses functional groups that act as barriers or interact with the guest molecule in a fashion that includes enantioselectivity. The most often used CSP of this type is CD bound to a silica support.
CDs are cyclic oligomers with 6 (\({\alpha}\)-CD), 7 (β-CD) or 8 (γ-CD) D-glucopyranose units through an (\({\alpha}\)-1,4- linkage that adopt a tapered cylindrical or toroidal shape (see Figure \(\PageIndex{4}\)). The toroid has a maximum diameter ranging from 5.7 Å (\({\alpha}\)-CD) to 9.5 Å (γ-CD) with a depth of about 7 Å. The exterior of the toroid is relatively hydrophilic because of the presence of the primary C-6 hydroxy groups at the smaller rim of the toroid and the secondary C-2 and C-3 hydroxy groups at the opposite end. The internal cavity is hydroxy-free and hydrophobic, which favors the enantioseparation of partially nonpolar compounds via selective inclusion. The modification of CDs and their complexation behavior involves substitution of one or more of the C-2, C-3 and C-6 hydroxy groups. The most commonly used derivatized CDs are sulfated, acetylated, permethylated, perphenylated, 2-hydroxypropylated, 3,5- dimethylcarbamoylated, and naphthylethyl-carbamoylated [73] CDs. Most of the studies involving CDs as CSPs in HPLC were accomplished in the RP mode, in the NP mode or in the polar-organic (PO) mode. Thus, CD-bonded CSPs are regarded as multi-modal phases.
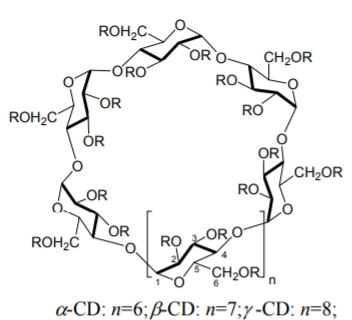
Figure \(\PageIndex{4}\): Structures of \({\alpha}\)-, β- and γ-CDs
A nother group of cavity based CSPs are formed using crown ethers, heteroatomic macrocycles with repeating (-X-C2H4-) units, where X, the heteroatom, is commonly oxygen, but may also be a sulfur or nitrogen atom. an example crown ether compound is shown in Figure \(\PageIndex{5}\). Unlike CDs, the host-guest interaction within the chiral cavity is hydrophilic in nature. Crown ethers, and especially 18-crown-6 ethers, can complex inorganic cations and alkylammonium compounds. This inclusion interaction is based mainly on H-bonding between the hydrogens of the ammonium group and the heteroatom of the crown ether. Additional electrostatic interaction occurs between the nitrogen and the crown ether’s oxygen lone pair electrons.
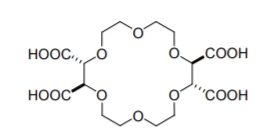
Figure \(\PageIndex{5}\): The structure of (+)-(18-crown-6)-2,3,11,12- tetracarboxylic acid.
Macrocyclic Antibiotic Phases
Macrocyclic antibiotics have proved to be an exceptionally useful class of chiral selectors for the separation of enantiomers of biological and pharmaceutical importance by means of HPLC, TLC and CE. The macrocyclic antibiotics are covalently bonded to silica gel via chains of different linkages, such as carboxylic acid, amine, epoxy or isocyanate-terminated organosilanes. This kind of attachment ensures the stability of the chiral selectors, while their chiral recognition properties are retained. All macrocyclic antibiotic stationary phases are multimodal CSPs, i.e. they can be used in normal phase, reverse phase, polar organic or polar-ionic separations. The antibiotics used for chiral separations in HPLC include the ansamycins (rifamycins), the glycopeptides (avoparcin, teicoplanin, ristocetin A, vancomycin and their analogs) and the polypeptide antibiotic thiostrepton. One of the most frequently used teicoplanin, Figure \(\PageIndex{6}\), consists of four fused macrocyclic rings, which contain seven aromatic rings, two of them bearing a chlorine substituent, and the others having a phenolic character.
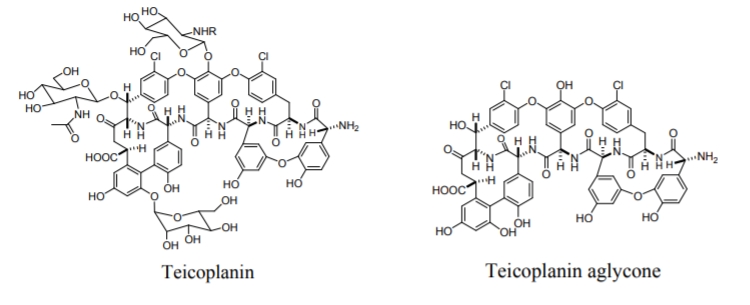
Figure \(\PageIndex{6}\): Structures of the macrocyclic glycopeptide teicoplanin and teicoplanin aglycone.

