9.5: Photometric and Spectrophotometric Titrations
- Page ID
- 220471
A photometric titration curve is a plot of absorbance, corrected for volume changes, as a function of the volume of titrant. If the conditions are properly chosen the curve will consisits of two linear regions of different slopes. One region occurs at the start of the titration and the other is well after the equivalence point. The end point is the volume of titrant corresponding to the point where the two linear regions intersect. A few examples of photometric titration curves are shown in Figure 9.5.1 where A indicates the analyte, P indicates the product and T indicates the titration for the reaction A + T --> P.
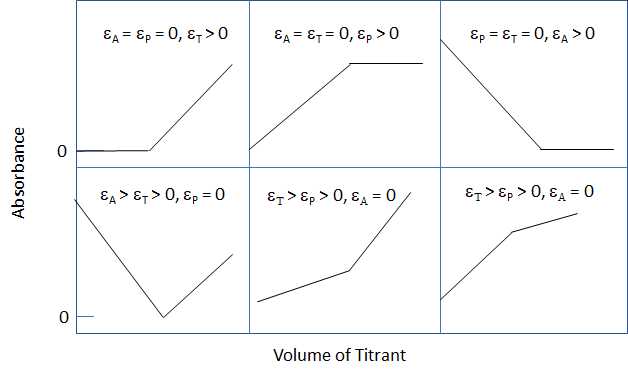
Figure\(\PageIndex {1}\): The illustration above shows some typical photmetric titration cuves where A indicates the analyte, T indicates the titrant, P indicates the product and \({\epsilon}\) indicates the molar absorptivity.
In order to obtain a satisfactory endpoint the absorbing system(s) must obey Beer's law and the absorbance values need be correct for the volume changes due to the addition of the volume of titrant; A' = A (V + v)/V where V is the original volume and v is the total volume of titrant added.

