9.3: Qualitative Applications of Ultraviolet Visible Absorption Spectroscopy
- Page ID
- 220469
In contrast to NMR and IR, spectroscopy in the UV and visible regions is of very limited in terms of the information content contained in a a molecule's spectrum. The small number of peaks, the broadness of the peaks makes the unambiguous identification of a compound from its UV-Vis spectrum very unlikely.
Methods of Plotting Spectra
UV/vis spectra are plotted in a number of different ways. The most common quantities plotted as the ordinate are absorbance, % transmittance, log absorbance, or molar absorptivity. The most common quantities plotted as the abscissa are wavelength, wavenumber (cm-1), or frequency.

Figure\(\PageIndex {1}\): Two plots of the absorbance spectrum of a 1.00 x 10-5 M solution of 1-naphthol in terms of (a) absorbance and (b) %transmittance.
Generally, plots in terms of absorbance give the largest difference in peak heights as a function of analyte concentration, the exception being for solution that do not absorb very much and where the curve to curve differences are larger with % transmittance.
Solvents
In addition to solubility, consideration must be given to the transparency of the solvent and the effect of the solvent on the spectrum of the analyte.
Any colorless solvent will be transparent in the visible region. For the UV, a low wavelength cutoff for common solvents can be easily found. This low wavelength cutoff is the wavelength below which the solvent absorbance increases to obscure the analyte's absorbance. A short table is given below
| Solvent | Low wavelength cutoff (nm) |
| water | 190 |
| ethanol | 210 |
| n-hexane | 195 |
| cyclohexane | 210 |
| benzene | 280 |
| diethyl ether | 210 |
| acetone | 310 |
| acetonitrile | 195 |
| carbon tetrachloride | 265 |
| methylene chloride | 235 |
| chloroform | 245 |
Spectral fine structure, often observed in the gas phase spectra, is either broadened or obscured by the solvent. In addition, the absorbance peak positions in a polar solvent are often shifted to longer wavelengths, red-shifted, relative to their position sin a non polar solvent. The shift in peak position can be as large as 40 nm and results from the stabilization or destabilization of the ground state and excited state energy levels by the solvent. Both the loss of fine structure and shift in peak positions is evident in the three spectra of flavone shown in Figure 9.3.2 obtained in methanol, acetonitrile, and cyclohexane. Focusing on the bands at around 290 nm the two bands (peak and shoulder) clearly observed in cyclohexane, the least polar solvent, is largely gone in methanol, the most polar solvent. Also a red shift on the order of 10 nm is evident for methanol relative to cyclhexane.
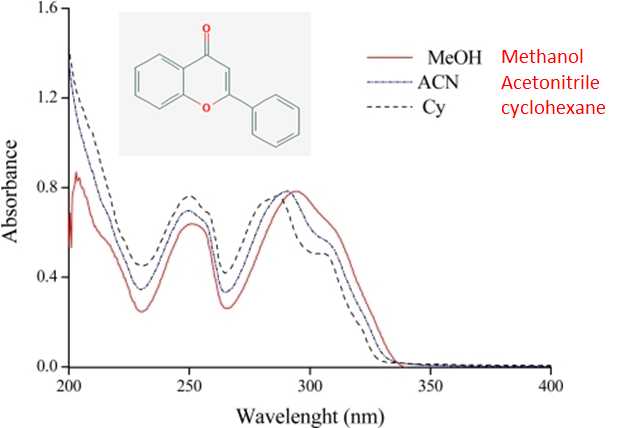
Figure\(\PageIndex {2}\): The UV spectrum of flavone in three different solvents. The spectra are from Spectroscopic Study of Solvent Effects on the Electronic Absorption Spectra of Flavone and 7-Hydroxyflavone in Neat and Binary Solvent Mixtures, Sancho, Amadoz, Blanco and Castro, Int. J. Mol. Sci. 2011, 12(12), 8895-8912

