5.2: Hydronium Ions
- Page ID
- 284487
A hydrogen ion, H+, is a hydrogen atom which has lost its single electron; that is, a hydrogen ion is just a proton. It does not, however, readily exist in an aqueous solution. Because a proton is only about one ten-thousandth as big as an average atom or ion, water dipoles can approach very close to a hydrogen ion in solution. Consequently the proton can exert a very strong attractive force on a lone pair of electrons in a water molecule—strong enough to form a coordinate covalent bond:
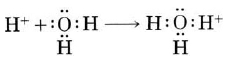
The H3O+ formed in this way is called a hydronium ion (on the left in the figure below). All three of its O―H bonds are exactly the same, and the ion has a pyramidal structure as predicted by VSEPR theory (1a). To emphasize the fact that a proton cannot exist by itself in aqueous solution, Eq. (1a) is often rewritten as
\[\text{2H}_{2}\text{O}(l) \rightleftharpoons \text{H}_{3}\text{O}^{+}(aq) + \text{OH}^{-}(aq)\]
Like other ions in aqueous solution, both hydronium and hydroxide ions are hydrated. Moreover, hydrogen bonds are involved in attracting water molecules to hydronium and hydroxide ions. In both cases three water molecules appear to be rather tightly held, giving formulas H3O(H2O)3+ (or H9O4+) and HO(H2O)3– (or H7O4–). Possible structures for the hydrated hydronium and hydroxide ions are shown in Figure \(\PageIndex{1}\).
Contributors
Ed Vitz (Kutztown University), John W. Moore (UW-Madison), Justin Shorb (Hope College), Xavier Prat-Resina (University of Minnesota Rochester), Tim Wendorff, and Adam Hahn.


