8.6: Oxidation and Reduction
- Page ID
- 315373
In some reactions, the products are formed because electrons migrate from one reactant to another. Because so many important reactions follow this pattern, a special vocabulary is used to talk about them. In general, they are known as redox reactions.
Oxidation and Reduction: Electron Transfer
Oxidation and reduction with respect to electron transfer
- Oxidation is loss of electrons
- Reduction is gain of electrons
Remembering these definitions is essential; this convenient acronym might be helpful:
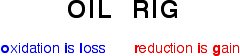
Example \(\PageIndex 1\)
The equation below shows an example of a redox reaction:
\[ \ce{ CuCl_2(aq) + Mg(s) \rightarrow Cu(s) + MgCl_2(aq)} \]
Copper(II) chloride and magnesium chloride are both ionic compounds. If the above is written as an ionic equation, it becomes apparent that the chloride ions are spectator ions. The net ionic equation gives:
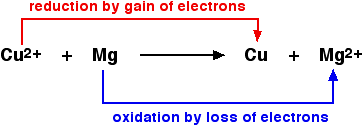
The copper(II) ion must gain two electrons the become a neutral copper atom. Since it gains electrons, copper is reduced in this reaction. The two electrons come from the magnesium atom. Since the magnesium atom loses two electrons when it becomes a magnesium ion, it is oxidized.
Oxidizing Agents and Reducing Agents
When talking about redox reactions, chemists often use the terms oxidizing agent and reducing agent.
- Oxidizing agents add electrons to another species. In the process, the oxidizing agent itself is reduced.
- Reducing agents remove electrons from another species. The reducing agent is oxidized for this to occur.
Example \(\PageIndex 2\)
What is the oxidizing agent in the reaction examined in example 1? The reducing agent?
\[ \ce{ Cu^2+(aq) + Mg(s) \rightarrow Cu(s) + Mg^2+(aq)} \]
We have already identified that the copper(II) ions are reduced in this reaction and that the elemental magnesium is oxidized. Since the copper(II) ions are reduced, they are the oxidizing agent. The elemental magnesium is the reducing agent.
Another way to think of this is that the copper(II) ions are the oxidizing agent because they cause the magnesium to lose electrons and become oxidized. Similarly, the elemental magnesium gives electrons to the copper(II) ions, forcing the copper(II) ions to reduce. As the agent of this change, the elemental magnesium is the reducing agent.
Half Reactions
For every redox reaction, two half reactions can be written showing the gain or loss of electrons explicitly. Unlike other ways of writing chemical equations you have seen so far, in half reactions, electrons appear as either reactants or products. The two half reactions for the reaction of copper(II) ions with elemental magnesium would be:
\[ \ce{ Mg(s) \rightarrow Mg^2+(aq) + 2e^-} \]
\[ \ce{ Cu^2+(aq) + 2e^- \rightarrow Cu(s) } \]
The first half reaction is the oxidation half reaction, since it shows the process of magnesium losing electrons. The second half reaction is the reduction half reaction, since it shows the copper(II) ions gaining electrons. Adding the two half reactions together gives the overall redox reaction.
Example \(\PageIndex 3\)
When chlorine gas is bubbled through a solution containing iron(II) ions, the ion(II) ions are converted to iron(III) ions. The chlorine gas is also coverted to chloride ions:
\[ \ce{ 2 Fe^2+(aq) + Cl2(g) \rightarrow 2 Fe^3+(aq) + 2 Cl^- (aq)} \]
What are the oxidation and reduction half reaction for this process?
Oxidation half reaction:
\[ \ce{ Fe^2+(aq) \rightarrow Fe^3+(aq) + e^- (aq)} \]
For an iron(II) ion to become and iron(III) ion, it has to lose one electron. This lost electron appears on the product side. Since an electron is being lost, this is an oxidation.
Reduction half reaction:
\[ \ce{Cl2(g) + 2 e^- (aq)\rightarrow 2 Cl^- (aq)} \]
A chlorine molecule contains two neutral chloride atoms. To become two chloride ions, the molecule must gain two electrons (each of the chloride atoms in the molecule gets one of the two electrons). Since chlorine is gaining electrons, this is a reduction.
To regain the original redox reaction, the reduction half reaction must be added to twice the oxidation half reaction. This is because the two electrons on the reactant side of the reduction half reaction need to cancel two electrons on the product side of the oxidation half reaction. The only way to get these two electrons in the oxidation half reaction is to double the whole equation.
Oxidation and Reduction: Oxygen Transfer
When talking about redox reactions involving organic molecules or biological molecules, alternative definitions of oxidation and reduction are often helpful. The terms oxidation and reduction can be defined in terms of the adding or removing oxygen to a compound. While this is not the most robust definition, it is the easiest to remember.
Oxidation and reduction with respect to oxygen transfer
- Oxidation is the gain of oxygen.
- Reduction is the loss of oxygen.
For example, in the extraction of iron from its ore:
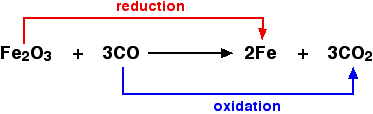
Since an oxidizing agent is substance which oxidizes something else, the iron(III) oxide is the oxidizing agent. Since a reducing agent reduces something else, the carbon monoxide is the reducing agent.
Unfortunately, these definitions of oxidation and reduction can not be applied to all redox reactions. For example, the reaction between copper(II) ions and elemental magnesium examined above is a redox reaction where using gain and loss of oxygen to define oxidation and reduction is impossible.
Oxidation and Reduction: Hydrogen Transfer
These are old definitions which are no longer used, except occasionally in organic chemistry.
Oxidation and reduction with respect to hydrogen transfer
- Oxidation is the loss of hydrogen.
- Reduction is the gain of hydrogen.
Notice that these are exactly the opposite of the oxygen definitions above.
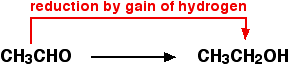
For example, ethanol can be oxidized to ethanal:

An oxidizing agent is required to remove the hydrogen from the ethanol. A commonly used oxidizing agent is potassium dichromate solution acidified with dilute sulfuric acid. Ethanal can also be reduced back to ethanol by adding hydrogen. A possible reducing agent is sodium tetrahydridoborate, NaBH4.
Contributors
Jim Clark (Chemguide.co.uk)
- Michael J. Mackel (Portland Community College)


