5.7: Polarity of Molecules
- Page ID
- 313567
Molecular Polarity of Diatomic Molecules
If there is only one bond in the molecule, the bond polarity determines the molecular polarity. Any diatomic molecule in which the two atoms are the same element must be a nonpolar molecule. A diatomic molecule that consists of a polar covalent bond, such as HF, is a polar molecule. A polar molecule is a molecule in which one end of the molecule is slightly positive, while the other end is slightly negative. The two electrically charged regions on either end of the molecule are called poles, similar to a magnet having a north and a south pole. Hence, a molecule with two poles is called a dipole. A simplified way to depict polar molecules like HF is pictured below (see figure below).
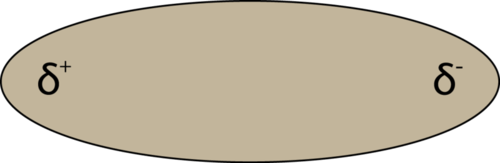
When placed between oppositely charged plates, polar molecules orient themselves so that their positive ends are closer to the negative plate and their negative ends are closer to the positive plate (see Figure 4.4.6 below).
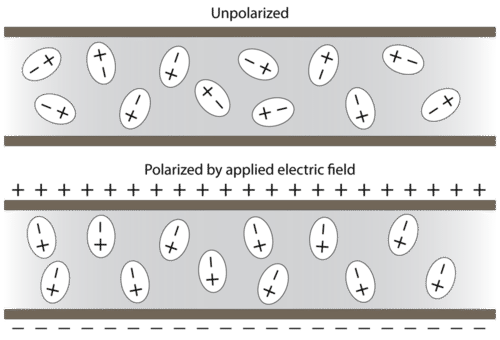
Experimental techniques involving electric fields can be used to determine if a certain substance is composed of polar molecules and to measure the degree of polarity.
Molecular Polarity of Larger Molecules
In general, a molecule is nonpolar if all its bonds are nonpolar. Examples are I2, O2, H2, CH4, C2H6 and C3H8.
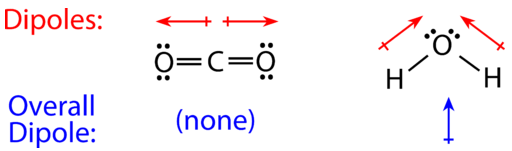
In general, a molecule is polar if it contains polar bonds EXCEPT when the bond polarities cancel each other. The shape of the CO2 molecule (linear) orients the two C=O polar bonds directly opposite each other, thus cancelling each other's effect. Carbon dioxide (CO2) is a nonpolar molecule.
On the other hand, water (as discussed above) is a bent molecule because of the two lone pairs on the central oxygen atom. Because of the bent shape, the dipoles do not cancel each other out and the water molecule is polar. In the figure below, the individual H-O polar bonds represented by the two red arrows are not directly opposite each other. These two dipoles don't cancel each other out. In fact, the net dipole (blue arrow) points upward. There is a resultant partial positive charge at one end (between the two H atoms) and a partial negative charge on the other end (where O is located). The uneven distribution of charge or the overall dipole is shown by the blue arrow below (Figure 4.5.1). Hence, water is polar (has + and - poles) while carbon dioxide is nonpolar.
Similarly, in BF3 (trigonal planar), the effect of a B-F bond is cancelled by the sum of the other two B-F bonds (see video). Hence, a trigonal planar molecule (BF3) is nonpolar because the bond polarities cancel each other, but a trigonal pyramidal molecule (NH3) is polar.

Some other molecules are shown in the figure below. Notice that a tetrahedral molecule such as CCl4 is nonpolar. However, if the peripheral atoms are not of the same electronegativity, the bond polarities don't cancel and the molecule becomes polar, as in CH3Cl.

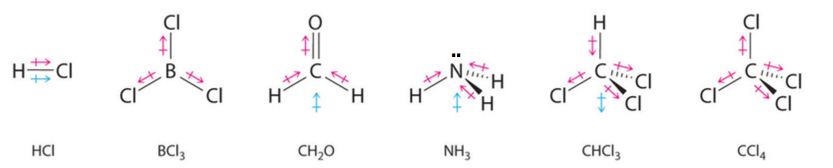
Figure \(\PageIndex{4}\): Molecules with Polar Bonds. Individual bond dipole moments are indicated in red. Due to their different three-dimensional geometry, some molecules with polar bonds have a net dipole moment (HCl, CH2O, NH3, and CHCl3), indicated in blue, whereas others do not because the bond dipoles cancel due to symmetry (BCl3 and CCl4).
Example \(\PageIndex{2}\)
Describe the shape of each molecule. Is it polar or nonpolar?
- PCl3
- CO2
Solution
- The Lewis diagram for PCl3 is as follows:
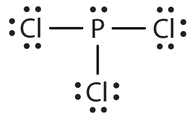
Focus on the central atom, P that has 3 bonds and one lone pair. The four electron pairs arrange themselves tetrahedrally, but the lone electron pair is not considered in describing the molecular shape. Like NH3, this molecule is pyramidal. The 3 P-Cl bonds don't cancel each other. This is polar.
- The Lewis diagram for CO2 is as follows:
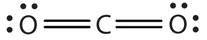
Focus on the central atom, C. The multiple bonds are treated as one group, hence C has 2 bonds and zero lone pair. CO2 has only two groups of electrons that repel each other. They will direct themselves 180° apart from each other, so CO2 molecules are linear. This is highly symmetrical, with the two opposite dipoles cancelling each other. The CO2 molecule is nonpolar.
Exercise \(\PageIndex{2}\)
Describe the shape of each molecule. Is it polar or nonpolar?
- CBr4
- BCl3
- Answer a:
-
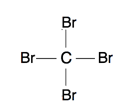 or
or 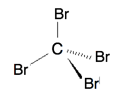
The Lewis structure shows 4 groups attached to the central atom, hence tetrahedral. All the 4 groups are identical and the shape is symmetrical. Hence, it is nonpolar.
- Answer b:
-

The Lewis diagram shows 3 groups attached to the central atom, hence trigonal planar. All the 3 groups are identical and shape is symmetrical, hence, it is nonpolar.
Exercises
-
Is each molecule polar or nonpolar? (Reminder: you determined their shapes in the exercises for the last section.)
- H2S
- COCl2
- SO2
-
Is each molecule polar or nonpolar? (Reminder: you determined their shapes in the exercises for the last section.)
- NBr3
- SF2
- SiH4
Answers
-
- polar
- polar
- polar
-
- polar
- polar
- nonpolar

