5.5: Molecular Geometry- VSEPR
- Page ID
- 313257
Unlike the ions in ionic compounds, which are arranged in lattices called crystals, molecules of covalent compounds exist as discrete units with a certain three-dimensional shape.
Molecular Shape: VSEPR Theory
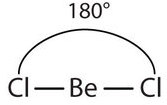
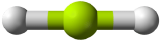
Unlike ionic compounds, with their extended crystal lattices, covalent molecules are discrete units with specific three-dimensional shapes. The shape of a molecule is determined by the fact that covalent bonds, which are composed of negatively charged electrons, tend to repel one another. This concept is called the valence shell electron pair repulsion (VSEPR) theory. For example, the two covalent bonds in \(\ce{BeCl2}\) stay as far from each other as possible, ending up 180° apart from each other. The result is a linear molecule:
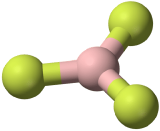
The three covalent bonds in BF3 repel each other to form 120° angles in a plane, in a shape called trigonal planar:

The molecules \(\ce{BeCl2}\) and \(\ce{BF3}\) actually violate the octet rule; however, such exceptions are rare.
Try sticking three toothpicks into a marshmallow or a gumdrop and see if you can find different positions where your “bonds” are farther apart than the planar 120° orientation.
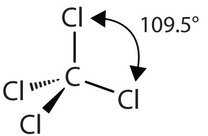
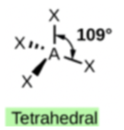
The four covalent bonds in CCl4 arrange themselves three dimensionally, pointing toward the corner of a tetrahedron and making bond angles of 109.5°. CCl4 is said to have a tetrahedral shape:
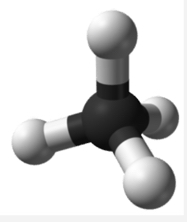
| Atoms Around Central Atom | Geometry | Example |
|---|---|---|
| 2 \(\ce{AB_2}\) | Linear | \(\ce{BeCl_2}\) |
| 3 \(\ce{AB_3}\) | Trigonal Planar | \(\ce{BF_3}\) |
| 4 \(\ce{AB_4}\) | Tetrahedral | \(\ce{CCl_4}\) |
In determining the shapes of molecules, it is useful to first determine the Lewis diagram for a molecule. The shapes of molecules with multiple bonds are determined by treating the multiple bonds as one bond. Thus, formaldehyde (CH2O) has a shape similar to that of BF3. It is trigonal planar.

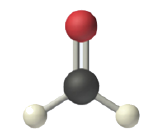
It is often useful to talk about both the electron geometry of the molecule and the molecular geometry. Electron geometry looks at the arrangement of all electron-rich areas (both lone pairs and covalent bonds) around the central atom. Molecular geometry looks at only the arrangement of covalent bonds. For molecules like BF3, CCl4, and CH2O in which the central atom has no lone pairs, the electron geometry and the molecular geometry are the same.
Molecules With Lone Pairs Around Central Atom
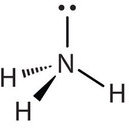
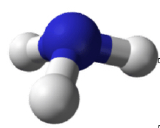
Molecules with lone electron pairs around the central atom have a molecular geometry based on the position of the atoms, not the electron pairs. For example, NH3 has one lone electron pair and three bonded electron pairs. These four electron pairs repel each other and adopt a tetrahedral electron geometry. However, the molecular geometry of the molecule is described in terms of the positions of the atoms, not the lone electron pairs. Thus, NH3 is said to have a trigonal pyramidal molecular geometry, not a tetrahedral one. Whenever there are lone electrons pairs on the central atom, the electron geometry and molecular geometry will be different.
Similarly, H2O has two lone pairs of electrons around the central oxygen atom and two bonded electron pairs. Although the four electron geometry is tetrahedral, the molecular geometry of the molecule is described by the positions of the atoms only. The molecular geometry of H2O is bent with an approximate 109.5° angle.
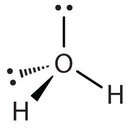
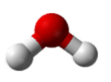
In summary, to determine the molecular geometry:
Step 1: Draw the Lewis structure.
Step 2: Count the number of bonds (a double/triple bond counts as one) and lone pairs around the central atom.
Step 3: Use Table 4.5.1 to determine the molecular geometry.

Table 4.5.1: The molecular geometry depends on the number of bonds and lone pairs around the central atom, A.
Example \(\PageIndex{1}\)
What is the electron geometry and molecular geometry of the ammonium ion, NH4+? Its Lewis structure is shown below. How is this different from ammonia, NH3?
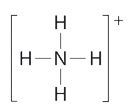
Solution
In ammonium ion, the central atom N has 4 bonds and no lone pairs. It is equivalent to the square shown below from Table 4.5.1. Hence, both the electron geometry and the molecular geometry are tetrahedral. Notice that determining the shape of a polyatomic ion is the same as determining the shape of a neutral molecule.
In ammonia (NH3), shown below, N has 3 bonds and one lone pair.
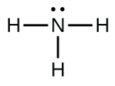
It is equivalent to the square shown below from Table 4.5.1. Hence, the molecular geometry of this molecule is trigonal pyramidal. The electron geometry, however, is tetrahedral.

Exercise \(\PageIndex{1}\)
What is the electron geometry and molecular geometry of nitrosyl chloride, a highly corrosive, reddish-orange gas? Its Lewis structure is shown below.
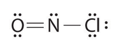
- Answer
-
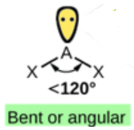
Focus on the central atom, N. It has a double bond to O, count this as one bond. It also has a single bond to Cl. Thus, N has 2 bonds and one lone pair. These 3 electron pairs will spread out 120 degrees from each other. This means the electron geometry will be trigonal planar. But, since the molcular geometry is defined by the arrangement of the atoms only, the shape is bent. If you consult Table 4.5.1, this molecule is equivalent to the below. Hence, two bonds and one lone pair has a bent shape.
Exercises
-
What is the electron geometry of each molecule? The molecular geometry?
- H2S
- COCl2
- SO2
-
What is the electron geometry of each molecule? The molecular geometry?
- NBr3
- SF2
- SiH4
-
Predict the molecular geometry of nitrous oxide (N2O), which is used as an anesthetic. A nitrogen atom is in the center of this three-atom molecule.
-
Predict the molecular geometry of acetylene (C2H2), which has the two carbon atoms in the middle of the molecule with a triple bond. What generalization can you make about the shapes of molecules that have more than one central atom?
Answers
-
- electron geometry: tetrahedral, molecular geometry: bent
- electron geometry: trigonal planar, molecular geometry: trigonal planar
- electron geometry: trigonal planar, molecular geometry: bent
-
- electron geometry: tetrahedral, molecular geometry: trigonal pyramidal
- electron geometry: tetrahedral, molecular geometry: bent
- electron geometry: tetrahedral, molecular geometry: tetrahedral
3. linear
4. linear; in a molecule with more than one central atom, the geometry around each central atom needs to be examined.

