6.2: Solutions Chemistry
- Page ID
- 217278
Skills to Develop
- Describe the basic properties of solutions and how they form
- Define and give examples of electrolytes
- Predict the solubility of common inorganic compounds by using solubility rules
- Write and balance chemical equations in molecular, total ionic, and net ionic formats.
The previous unit introduced solutions, defined as homogeneous mixtures of two or more substances. Often, one component of a solution is present at a significantly greater concentration, in which case it is called the solvent. The other components of the solution present in relatively lesser concentrations are called solutes. Sugar is a covalent solid composed of sucrose molecules, \(\mathrm{C_{12}H_{22}O_{11}}\). When this compound dissolves in water, its molecules become uniformly distributed among the molecules of water:
\[\mathrm{C_{12}H_{22}O}_{11(s)}⟶\mathrm{C_{12}H_{22}O}_{11(aq)} \label{Eq1}\]
The subscript “aq” in the equation signifies that the sucrose molecules are solutes and are therefore individually dispersed throughout the aqueous solution (water is the solvent). Although sucrose molecules are heavier than water molecules, they remain dispersed throughout the solution; gravity does not cause them to “settle out” over time.
Potassium dichromate, \(\mathrm{K_2Cr_2O_7}\), is an ionic compound composed of colorless potassium ions, \(\mathrm{K^+}\), and orange dichromate ions, \(\mathrm{Cr_2O_7^{2−}}\)
\[\mathrm{K_2Cr_2O}_{7(s)}⟶\mathrm{2K^+}_{(aq)}+\mathrm{Cr_2O_7^{2-}}_{(aq)} \label{Eq2}\]
As with the mixture of sugar and water, this mixture is also an aqueous solution. Its solutes, potassium and dichromate ions, remain individually dispersed among the solvent (water) molecules.

Figure \(\PageIndex{1}\): When potassium dichromate (\(K_2Cr_2O_7\)) is mixed with water, it forms a homogeneous orange solution. (credit: modification of work by Mark Ott).
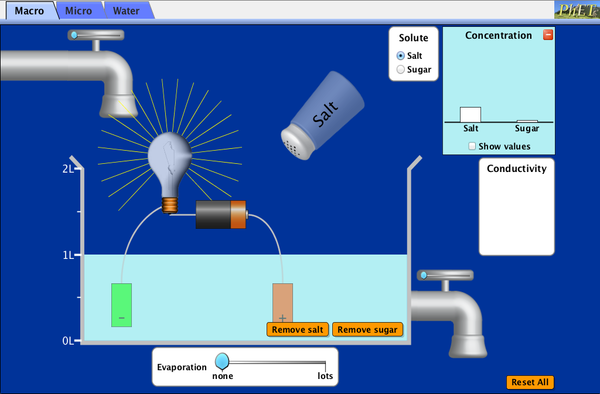
Learn More about The dissolution of covalent and ionic compounds
Click the link to use a simulator.
Water is used so often as a solvent that the word solution has come to imply an aqueous solution to many people. However, almost any gas, liquid, or solid can act as a solvent. Many alloys are solid solutions of one metal dissolved in another; for example, US five-cent coins contain nickel dissolved in copper. Air is a gaseous solution, a homogeneous mixture of nitrogen, oxygen, and several other gases. Oxygen (a gas), alcohol (a liquid), and sugar (a solid) all dissolve in water (a liquid) to form liquid solutions. Table \(\PageIndex{1}\) gives examples of several different solutions and the phases of the solutes and solvents.
| Solution | Solute | Solvent |
|---|---|---|
| air | O2(g) | N2(g) |
| soft drinks | CO2(g) | H2O(l) |
| hydrogen in palladium | H2(g) | Pd(s) |
| rubbing alcohol | H2O(l) | C3H8O(l) (2-propanol) |
| saltwater | NaCl(s) | H2O(l) |
| brass | Zn(s) | Cu(s) |
Solutions exhibit these defining traits:
- They are homogeneous; that is, after a solution is mixed, it has the same composition at all points throughout (its composition is uniform).
- The physical state of a solution—solid, liquid, or gas—is typically the same as that of the solvent, as demonstrated by the examples in Table \(\PageIndex{1}\).
- The components of a solution are dispersed on a molecular scale; that is, they consist of a mixture of separated molecules, atoms, and/or ions.
- The dissolved solute in a solution will not settle out or separate from the solvent.
- The composition of a solution, or the concentrations of its components, can be varied continuously, within limits.
Electrolytes & Nonelectrolytes
When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. These substances constitute an important class of compounds called electrolytes. Substances that do not yield ions when dissolved are called nonelectrolytes. If the physical or chemical process that generates the ions is essentially 100% efficient (all of the dissolved compound yields ions), then the substance is known as a strong electrolyte. If only a relatively small fraction of the dissolved substance undergoes the ion-producing process, it is called a weak electrolyte.
Substances may be identified as strong, weak, or nonelectrolytes by measuring the electrical conductance of an aqueous solution containing the substance. To conduct electricity, a substance must contain freely mobile, charged species. Most familiar is the conduction of electricity through metallic wires, in which case the mobile, charged entities are electrons. Solutions may also conduct electricity if they contain dissolved ions, with conductivity increasing as ion concentration increases. Applying a voltage to electrodes immersed in a solution permits assessment of the relative concentration of dissolved ions, either quantitatively, by measuring the electrical current flow, or qualitatively, by observing the brightness of a light bulb included in the circuit (Figure \(\PageIndex{6}\)).
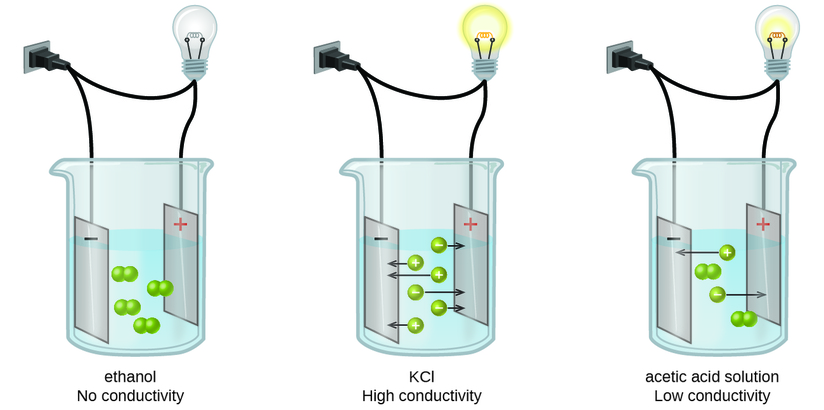
Figure \(\PageIndex{2}\): Solutions of nonelectrolytes such as ethanol do not contain dissolved ions and cannot conduct electricity. Solutions of electrolytes contain ions that permit the passage of electricity. The conductivity of an electrolyte solution is related to the strength of the electrolyte.
Ionic Electrolytes
Water and other polar molecules are attracted to ions, as shown in Figure \(\PageIndex{2}\).
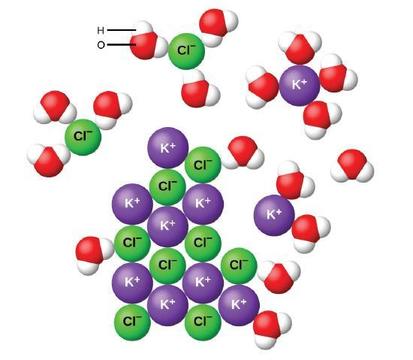
Figure \(\PageIndex{3}\): As potassium chloride (KCl) dissolves in water, the ions are hydrated. The polar water molecules are attracted by the charges on the K+ and Cl− ions. Water molecules in front of and behind the ions are not shown.
When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the strong electrostatic forces between them. This process represents a physical change known as dissociation. Under most conditions, ionic compounds will dissociate nearly completely when dissolved, and so they are classified as strong electrolytes.
Let us consider what happens at the microscopic level when we add solid KCl to water. Forces attract the positive (hydrogen) end of the polar water molecules to the negative chloride ions at the surface of the solid, and they attract the negative (oxygen) ends to the positive potassium ions. The water molecules penetrate between individual K+ and Cl− ions and surround them, reducing the strong interionic forces that bind the ions together and letting them move off into solution as solvated ions, as Figure shows. The reduction of the electrostatic attraction permits the independent motion of each hydrated ion in a dilute solution, resulting in an increase in the disorder of the system as the ions change from their fixed and ordered positions in the crystal to mobile and much more disordered states in solution. This increased disorder is responsible for the dissolution of many ionic compounds, including KCl, which dissolve with absorption of heat.
In other cases, the electrostatic attractions between the ions in a crystal are so large that the increase in disorder cannot compensate for the energy required to separate the ions, and the crystal is insoluble. Such is the case for compounds such as calcium carbonate (limestone), calcium phosphate (the inorganic component of bone), and iron oxide (rust).
Covalent Electrolytes
Pure water is an extremely poor conductor of electricity because it is only very slightly ionized—only about two out of every 1 billion molecules ionize at 25 °C. Water ionizes when one molecule of water gives up a proton to another molecule of water, yielding hydronium and hydroxide ions.
\[\ce{H_2O (l)+ H_2O (l) \rightleftharpoons H_3O^{+} (aq) + OH^{−} (aq)} \label{11.3.2}\]
In some cases, we find that solutions prepared from covalent compounds conduct electricity because the solute molecules react chemically with the solvent to produce ions. For example, pure hydrogen chloride is a gas consisting of covalent HCl molecules. This gas contains no ions. However, when we dissolve hydrogen chloride in water, we find that the solution is a very good conductor. The water molecules play an essential part in forming ions: Solutions of hydrogen chloride in many other solvents, such as benzene, do not conduct electricity and do not contain ions.
Hydrogen chloride is an acid, and so its molecules react with water, transferring H+ ions to form hydronium ions (\(H_3O^+\)) and chloride ions (Cl−):
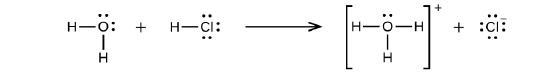
This reaction is essentially 100% complete for HCl (i.e., it is a strong acid and, consequently, a strong electrolyte). Likewise, weak acids and bases that only react partially generate relatively low concentrations of ions when dissolved in water and are classified as weak electrolytes. The reader may wish to review the discussion of strong and weak acids provided in the earlier chapter of this text on reaction classes and stoichiometry.
Solubility
Solubility is the tendency of one substance to dissolve in another. In chemistry, the term solubility is often used to denote a substance's aqueous solubility, its ability to dissolve in water.In general if a substance does not dissolve in water it is labeled insoluble and if it does dissolve in water it is called as soluble. In fact, solubility is much more nuanced than the general terms soluble and insoluble describe. These nuances will be explored later in this course, for now, we will describe substances as soluble or insoluble.
A substance's solubility is determined by several factors, including the types and relative strengths of intermolecular attractive forces that may exist between the substances’ atoms, ions, or molecules. For example, oil and water do not mix because the intermolecular forces that attract oil particles to one another and the intermolecular forces that attract water molecules together are much stronger than the intermolecular forces that attract oil particles to water molecules. Intermolecular forces is a subject that will be expanded on in greater detail later in this course.
Video \(\PageIndex{1}\): An overview of solubility.
Aqueous Solubility of Ionic Compounds
When studying chemical reactions, it is important to determine if a substance is soluble or insoluble in the reaction solvent. The next sections in this unit will cover common chemical reactions in aqueous solutions, therefore we must learn how to assess the soluble of substances in water. Table 6.2.1 outlines a set of rules that have been written based upon empirical evidence.
Table \(\PageIndex{1}\): Solubilities of Common Ionic Compounds in Water
| Soluble compounds contain | Exceptions to these solubility rules include |
|
|
| Insoluble compounds contain | Exceptions to these insolubility rules include |
|
|
Representing Aqueous Reactions
Given the abundance of water on earth, it stands to reason that a great many chemical reactions take place in aqueous media. When ions are involved in these reactions, the chemical equations may be written with various levels of detail appropriate to their intended use. To illustrate this, consider a reaction between ionic compounds taking place in an aqueous solution. When aqueous solutions of \(\ce{CaCl2}\) and \(\ce{AgNO3}\) are mixed, a reaction takes place producing aqueous \(\ce{Ca(NO3)2}\) and solid \(\ce{AgCl}\):
This balanced equation, derived in the usual fashion, is called a molecular equation because it doesn’t explicitly represent the ionic species that are present in solution. When ionic compounds dissolve in water, they may dissociate into their constituent ions, which are subsequently dispersed homogenously throughout the resulting solution (a thorough discussion of this important process is provided in the chapter on solutions). Ionic compounds dissolved in water are, therefore, more realistically represented as dissociated ions, in this case:
\[\ce{CaCl2}(aq)\rightarrow \ce{Ca^2+}(aq)+\ce{2Cl-}(aq)\]
\[\ce{2AgNO3}(aq)\rightarrow \ce{2Ag+}(aq)+\ce{2NO3-}(aq)\]
\[\ce{Ca(NO3)2}(aq)\rightarrow \ce{Ca^2+}(aq)+\ce{2NO3-}(aq)\]
Unlike these three ionic compounds, AgCl does not dissolve in water to a significant extent, as signified by its physical state notation, (s).
Explicitly representing all dissolved ions results in a complete ionic equation. In this particular case, the formulas for the dissolved ionic compounds are replaced by formulas for their dissociated ions:
\[\ce{Ca^2+}(aq)+\ce{2Cl-}(aq)+\ce{2Ag+}(aq)+\ce{2NO3-}(aq)\rightarrow \ce{Ca^2+}(aq)+\ce{2NO3-}(aq)+\ce{2AgCl}(s)\]
Examining this equation shows that two chemical species are present in identical form on both sides of the arrow, \(\ce{Ca^{2+}(aq)}\) and
\[\cancel{\ce{Ca^2+}(aq)}+\ce{2Cl-}(aq)+\ce{2Ag+}(aq)+\cancel{\ce{2NO3-}(aq)}\rightarrow \cancel{\ce{Ca^2+}(aq)}+\cancel{\ce{2NO3-}(aq)}+\ce{2AgCl}(s)\]
\[\ce{2Cl-}(aq)+\ce{2Ag+}(aq)\rightarrow \ce{2AgCl}(s)\]
Following the convention of using the smallest possible integers as coefficients, this equation is then written:
\[\ce{Cl-}(aq)+\ce{Ag+}(aq)\rightarrow \ce{AgCl}(s)\]
This net ionic equation indicates that solid silver chloride may be produced from dissolved chloride and silver(I) ions, regardless of the source of these ions. These molecular and complete ionic equations provide additional information, namely, the ionic compounds used as sources of \(\ce{Cl^{−}}\) and \(\ce{Ag+}\).
Example \(\PageIndex{1}\): Molecular and Ionic Equations
When carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium carbonate and liquid water. Write balanced molecular, complete ionic, and net ionic equations for this process.
Solution
Begin by identifying formulas for the reactants and products and arranging them properly in chemical equation form:
Balance is achieved easily in this case by changing the coefficient for NaOH to 2, resulting in the molecular equation for this reaction:
\[\ce{CO2(aq)+2NaOH(aq)\rightarrow Na2CO3(aq) + H2O}(l) \nonumber\]
The two dissolved ionic compounds, NaOH and Na2CO3, can be represented as dissociated ions to yield the complete ionic equation:
Finally, identify the spectator ion(s), in this case Na+(aq), and remove it from each side of the equation to generate the net ionic equation:
\[\begin{align*}
\ce{CO2}(aq)+\cancel{\ce{2Na+}(aq)}+\ce{2OH-}(aq)&\rightarrow \cancel{\ce{2Na+}(aq)}+\ce{CO3^2-}(aq)+\ce{H2O}(l)\\
\ce{CO2}(aq)+\ce{2OH-}(aq)&\rightarrow \ce{CO3^2-}(aq)+\ce{H2O}(l)
\end{align*}\]
Exercise \(\PageIndex{1}\)
Diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with diatomic hydrogen, via the electrolysis of brine, according to the following unbalanced equation:
\[\ce{NaCl(aq) + H2O(l) ->[ electricity] NaOH(aq) + H2(g) + Cl2(g)} \nonumber\]
Write balanced molecular, complete ionic, and net ionic equations for this process.
- Answer
-
Balanced molecular equation: \[\ce{2NaCl (aq) + 2H2O(l) \rightarrow 2NaOH (aq) + H2(g) + Cl2(g)} \nonumber\]
Balanced ionic equation: \[\ce{2Na^{+}(aq) + 2Cl^{-}(aq) + 2H2O(l) \rightarrow 2Na^{+}(aq) + 2OH^{-}(aq) + H2(g) + Cl2(g)} \nonumber\]
Balanced net ionic equation: \[\ce{2Cl^{-}(aq) + 2H2O(l) \rightarrow 2OH^{-}(aq) + H2(g) + Cl2 (g) } \nonumber\]
Summary
Video \(\PageIndex{5}\): An overview of solutions and electrolytes.
A solution forms when two or more substances combine physically to yield a mixture that is homogeneous at the molecular level. The solvent is the most concentrated component and determines the physical state of the solution. The solutes are the other components typically present at concentrations less than that of the solvent. Solutions may form endothermically or exothermically, depending upon the relative magnitudes attractive forces we will discuss in the second term of this course. Ideal solutions form with no appreciable change in energy.
Substances that dissolve in water to yield ions are called electrolytes. Electrolytes may be covalent compounds that chemically react with water to produce ions (for example, acids and bases), or they may be ionic compounds that dissociate to yield their constituent cations and anions, when dissolved. Soluble ionic substances and strong acids ionize completely and are strong electrolytes, while weak acids and bases ionize to only a small extent and are weak electrolytes. Nonelectrolytes are substances that do not produce ions when dissolved in water.
Glossary
- alloy
- solid mixture of a metallic element and one or more additional elements
- dissociation
- physical process accompanying the dissolution of an ionic compound in which the compound’s constituent ions are solvated and dispersed throughout the solution
- complete ionic equation
- chemical equation in which all dissolved ionic reactants and products, including spectator ions, are explicitly represented by formulas for their dissociated ions
- electrolyte
- substance that produces ions when dissolved in water
- insoluble
- of relatively low solubility; dissolving only to a slight extent
- molecular equation
- chemical equation in which all reactants and products are represented as neutral substances
- net ionic equation
- chemical equation in which only those dissolved ionic reactants and products that undergo a chemical or physical change are represented (excludes spectator ions)
- nonelectrolyte
- substance that does not produce ions when dissolved in water
- soluble
- of relatively high solubility; dissolving to a relatively large extent
- solubility
- the extent to which a substance may be dissolved in water, or any solvent
- strong electrolyte
- substance that dissociates or ionizes completely when dissolved in water
- supersaturated
- of concentration that exceeds solubility; a nonequilibrium state
- weak electrolyte
- substance that ionizes only partially when dissolved in water
Contributors
Paul Flowers (University of North Carolina - Pembroke), Klaus Theopold (University of Delaware) and Richard Langley (Stephen F. Austin State University) with contributing authors. Textbook content produced by OpenStax College is licensed under a Creative Commons Attribution License 4.0 license. Download for free at http://cnx.org/contents/85abf193-2bd...a7ac8df6@9.110).
- Adelaide Clark, Oregon Institute of Technology
- Crash Course Chemistry: Crash Course is a division of Complexly and videos are free to stream for educational purposes.
- TED-Ed’s commitment to creating lessons worth sharing is an extension of TED’s mission of spreading great ideas. Within TED-Ed’s growing library of TED-Ed animations, you will find carefully curated educational videos, many of which represent collaborations between talented educators and animators nominated through the TED-Ed website.