Following the work of Ernest Rutherford and his colleagues in the early twentieth century, the picture of atoms consisting of tiny dense nuclei surrounded by lighter and even tinier electrons continually moving about the nucleus was well established. This picture was called the planetary model, since it pictured the atom as a miniature “solar system” with the electrons orbiting the nucleus like planets orbiting the sun. The simplest atom is hydrogen, consisting of a single proton as the nucleus about which a single electron moves. The electrostatic force attracting the electron to the proton depends only on the distance between the two particles, based on Coulomb's Law:
\[ F_{gravity} = G \dfrac{ m_1 m_2}{r^2} \]
with
- \(G\) is a gravitational constant
- \(m_1\) and \(m_2\) are the masses of particle 1 and 2, respectively
- \(r\) is the distance between the two particles
The electrostatic force has the same form as the gravitational force between two mass particles except that the electrostatic force depends on the magnitudes of the charges on the particles (+1 for the proton and −1 for the electron) instead of the magnitudes of the particle masses that govern the gravitational force.
\[ F_{electrostatic} = k \dfrac{ m_1 m_2}{r^2}\]
with
- \(k\) is a constant
- \(m_1\) and \(m_2\) are the masses of particle 1 and 2, respectively
- \(r\) is the distance between the two particles
The electrostatic force is a vector quantity and is expressed in units of newtons. The force is understood to be along the line joining the two charges. (Figure \(\PageIndex{2}\))
Although the formula for Coulomb’s law is simple, it was no mean task to prove it. The experiments Coulomb did, with the primitive equipment then available, were difficult. Modern experiments have verified Coulomb’s law to great precision. For example, it has been shown that the force is inversely proportional to distance between two objects squared \((F\propto 1/r^{2})\) to an accuracy of 1 part in \(10^{16}\). No exceptions have ever been found, even at the small distances within the atom.
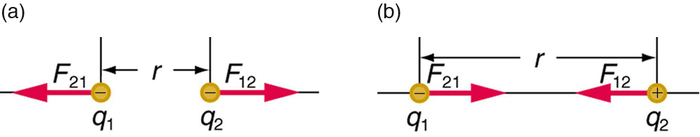
Figure \(\PageIndex{2}\): The magnitude of the electrostatic force\(F\) between point charges \(q_{1}\) and \(q_{2}\) separated by a distance \(r\) is given by Coulomb’s law. Note that Newton’s third law (every force exerted creates an equal and opposite force) applies as usual—the force on \(q_{1}\) is equal in magnitude and opposite in direction to the force it exerts on \(q_{2}\). (a) Like charges. (b) Unlike charges.
Since forces can be derived from potentials, it is convenient to work with potentials instead, since they are forms of energy. The electrostatic potential is also called the Coulomb potential. Because the electrostatic potential has the same form as the gravitational potential, according to classical mechanics, the equations of motion should be similar, with the electron moving around the nucleus in circular or elliptical orbits (hence the label “planetary” model of the atom). Potentials of the form V(r) that depend only on the radial distance \(r\) are known as central potentials. Central potentials have spherical symmetry, and so rather than specifying the position of the electron in the usual Cartesian coordinates (x, y, z), it is more convenient to use polar spherical coordinates centered at the nucleus, consisting of a linear coordinate r and two angular coordinates, usually specified by the Greek letters theta (θ) and phi (Φ). These coordinates are similar to the ones used in GPS devices and most smart phones that track positions on our (nearly) spherical earth, with the two angular coordinates specified by the latitude and longitude, and the linear coordinate specified by sea-level elevation. Because of the spherical symmetry of central potentials, the energy and angular momentum of the classical hydrogen atom are constants, and the orbits are constrained to lie in a plane like the planets orbiting the sun. This classical mechanics description of the atom is incomplete, however, since an electron moving in an elliptical orbit would be accelerating (by changing direction) and, according to classical electromagnetism, it should continuously emit electromagnetic radiation. This loss in orbital energy should result in the electron’s orbit getting continually smaller until it spirals into the nucleus, implying that atoms are inherently unstable.



