Many enzymes that catalyze reactions involving the phosphate diester bonds of DNA have been harnessed for use in genetic engineering - techniques in which we copy, snip, and splice DNA in order to create custom versions of genes. The tools of genetic engineering have become indispensable and commonplace in the past decade, and most researchers working on the biological side of chemistry use them extensively. The days of painstakingly purifying an enzyme from bacterial cultures or ground-up cow livers are pretty much gone. Now scientists clone the gene that encodes the enzyme, make any desired changes (by site-directed mutagenesis, for example), and use a host such as E. coli or yeast to produce 'recombinant' enzyme from the cloned gene. You will learn the details of many of these procedures in a biochemistry or molecular biology course. What we will focus on now is applying what we have learned about phosphate group transfer reactions so that we can recognize some of the organic chemistry that is happening in a cloning experiment.
The first thing you have to do in a gene cloning procedure is to copy a DNA strand. This is accomplished by an enzyme called DNA polymerase (EC 2.7.7.7), which uses a single strand of DNA as a template to synthesize a second, complementary strand (the full picture of this complex process is well beyond the scope of this book, but recall that we talked about the discovery of thermostable DNA polymerase in the introduction to chapter 6).
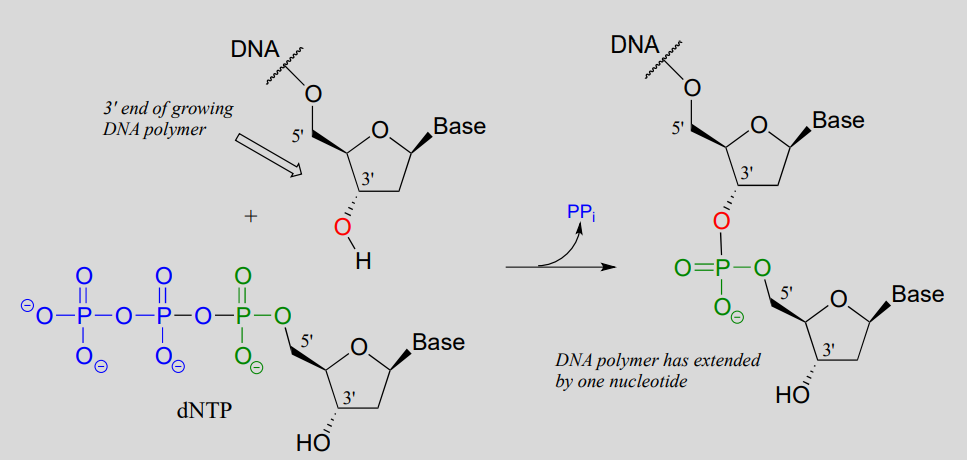
may have learned in a biology class that DNA is synthesized in the 3' to 5' direction. Notice below that the 3' hydroxyl group on the end of the growing DNA strand attacks the a-phosphate of a 2'-deoxynucleoside triphosphate (dNTP), expelling inorganic pyrophosphate.
DNA polymerase reaction:
Scientists are able to cut DNA using 'molecular scissor' enzymes called restriction endonucleases that cleave double-stranded DNA by hydrolysis at specific base sequences.
DNA hydrolysis by restriction endonucleases:
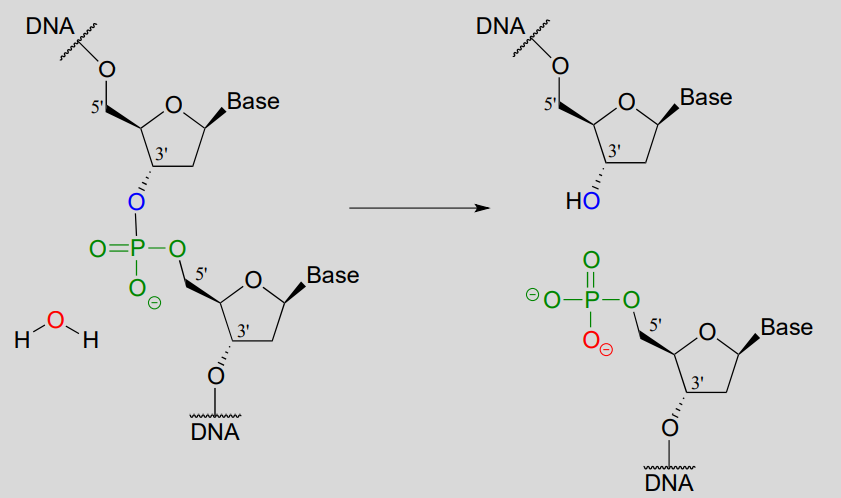
Notice that the result of this hydrolytic cleavage reaction is one segment of DNA with a hydroxy group at the 3' position, and a second segment with a phosphate group at the 5' position.
A commonly used restriction endonuclease called 'BamHI' cleaves double-stranded DNA specifically at the following 6-base sequence:
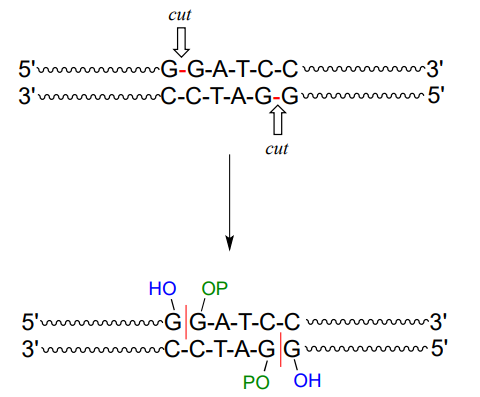
Notice that a 'staggered' cut is made: this is a common (and useful) property of many endonucleases, although some make 'blunt-ended' cuts.
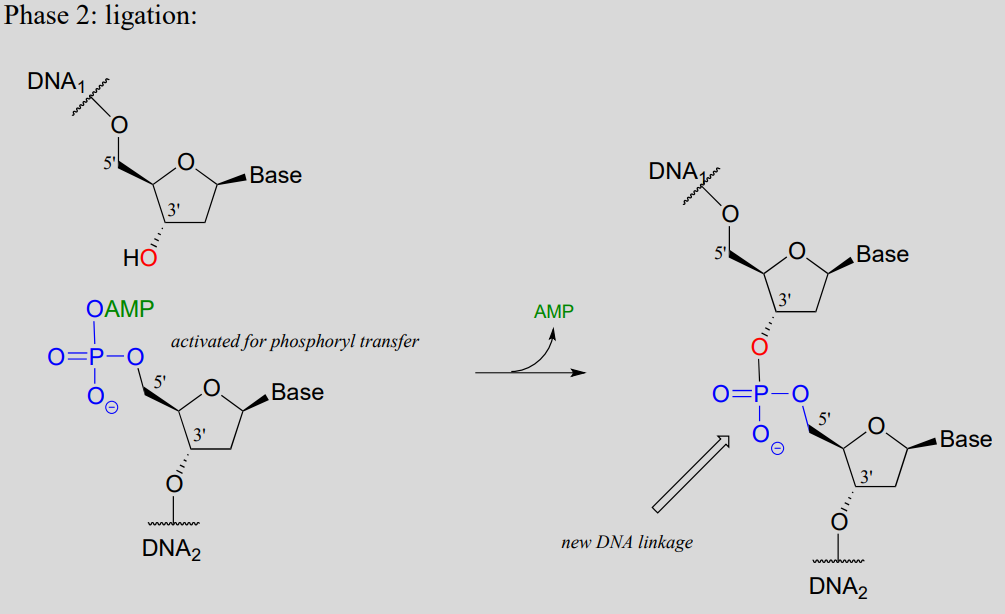
While an endonuclease cleaves a phosphodiester linkage in a DNA strand, DNA ligase (EC 6.5.1.1) accomplishes the reverse process: it catalyzes the formation of a new 3'-5' link between two strands:
DNA ligase reaction:
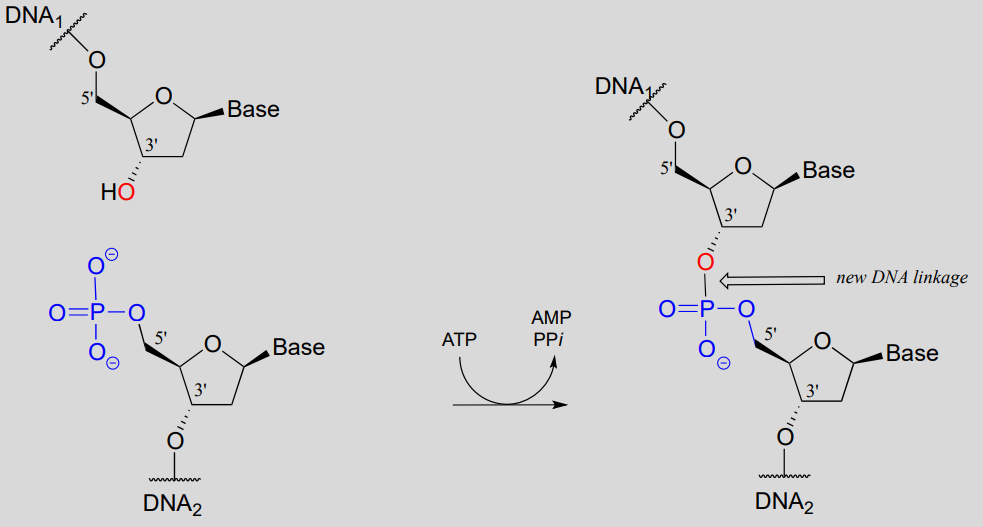
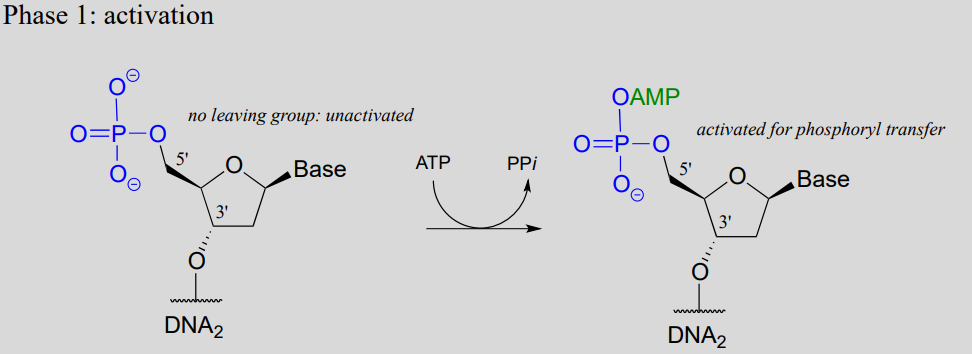
Note that there is initially no leaving group on the 5' phosphate of DNA2, which makes a direct phosphate transfer reaction impossible. The strategy employed by the DNA ligase enzyme is to first activate the 5' phosphate of DNA2 using ATP (phase 1 below), then the ligation reaction can proceed (phase 2)
DNA ligation
One more enzymatic tool in the genetic engineering arsenal bears mention. In some cloning procedures, a researcher may want to prevent unwanted ligation of DNA. This can be accomplished by using the enzyme alkaline phosphatase (EC 3.1.3.1), which catalyzes the dephosphorylation of many different organic phosphates, including 5'-phosphorylated DNA (recall that we discussed phosphatases in section 9.6).
Alkaline phosphatase reaction:
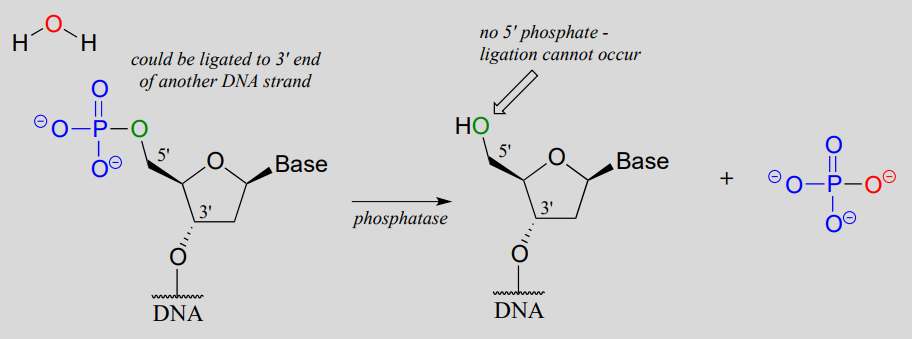
With the phosphate group removed, ligation is impossible - there is no way to make a new phosphodiester bond without a 5' phosphate group!









