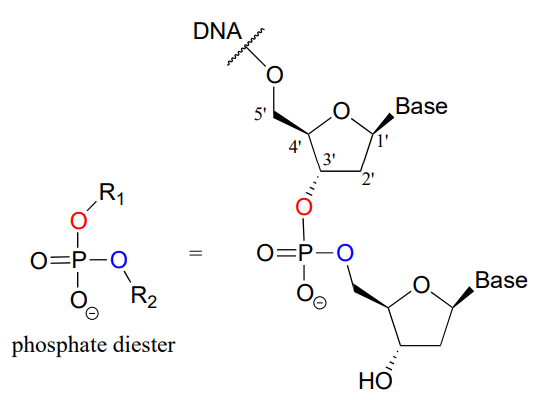
Phosphate diesters play an absolutely critical role in nature - they are the molecular 'tape' that connect the individual nucleotides in DNA and RNA via a sugar-phosphate backbone. Take note of the 1' - 5' carbon numbering shown below for the ribose sugar - these numbers will be used frequently in the coming discussion. The 'prime' symbol (') is used to distinguish the ribose carbon numbers from the nucleotide base carbon numbers (which are not shown here).
The introduction to this chapter referenced a widely-read 1987 commentary in Science Magazine, in which F.H. Westheimer of Harvard University addressed the question of why phosphates were 'chosen' by nature for critical biochemical job of linking DNA (Science 1987, 235, 1173). He emphasizes how critical it is for the phosphate diester linkage in DNA to be stable in water – in other words, it must be resistant to spontaneous (nonenzymatic) hydrolysis. Even very infrequent occurrence of such an undesired hydrolysis event could be disastrous for an organism, given that an intact DNA strand is a long-term storage mechanism for genetic information.
Westheimer pointed out that the inherent stability of DNA is a due in large part to the negative charge on the non-bridging oxygen of the phosphate diester linker, which effectively repels nucleophilic water molecules and shields the electrophilic phosphorus atom from attack.
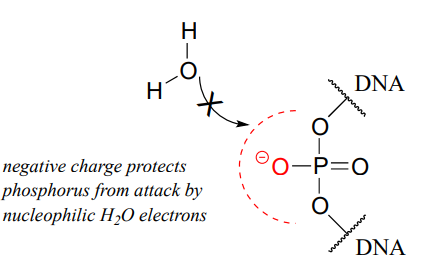
While DNA is quite stable with regard to spontaneous hydrolysis, it of course can be degraded by specific enzymatic hydrolysis, where the phosphate electrophile is activated for attack through noncovalent interactions (eg. with \(Mg^{2+}\)) in the active site. Enzymes that hydrolyze the phosphate diester bonds in DNA are called nucleases, and we will learn more about them in section 9.8.
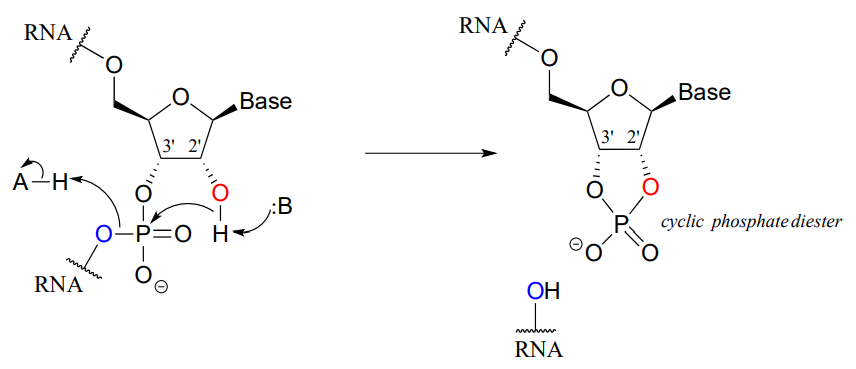
Unlike DNA, RNA is quite vulnerable to spontaneous hydrolysis in aqueous solution. This does not present a physiological dilemma, because the function of RNA is to encode genetic information on a temporary rather than long-term basis. Why does hydrolysis occur so much more rapidly in RNA than in DNA? The answer has everything to do with the lowered entropic barrier to the reaction (you might want to quickly review the concept of entropy at this point). RNA nucleotides, unlike the deoxynucleotides of DNA, have a hydroxyl group at the neighboring 2' carbon. The 2' hydroxyl group is right next to the electrophilic phosphorus atom, poised in a good position to make a nucleophilic attack, breaking the RNA chain and forming a cyclic phosphate diester intermediate (see figure below).
Researchers working with RNA have to be careful to store their samples at very cold temperatures, preferably freeze-dried or precipitated in ethanol, to avoid hydrolysis. The problem of RNA decomposition is compounded by the fact that RNAase enzymes, which catalyze RNA hydrolysis, are present on the surface of human skin and are very stable, long-lived, and difficult to destroy.
In contrast, DNA samples can be safely stored in aqueous buffer in a refrigerator, or in a freezer for longer-term storage.





