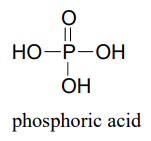
This chapter is about the chemistry of phosphates, a ubiquitous functional group in biomolecules that is based on phosphoric acid:
In late 2010, people around the world found themselves getting a crash course in phosphate chemistry as they watched the evening news. Those who paid close attention to the developing story also got an interesting glimpse into the world of scientific research and debate.
It all started when the American National Aeronautics and Space Administration (NASA) released the following statement to the news media:
“NASA will hold a news conference at 2 p.m. EST on Thursday, Dec. 2, to discuss an astrobiology finding that will impact the search for evidence of extraterrestrial life.”
The wording of the statement attracted widespread media attention, and had some people holding their breath in anticipation that NASA would be introducing a newly discovered alien life form to the world. When December 2nd came, however, those hoping to meet ET were disappointed – the life form being introduced was a bacterium, and it was from our own planet. To biologists and chemists, though, the announcement was nothing less than astounding.
The NASA scientists worked hard to emphasize the significance of their discovery during the news conference. Dr. Felicia Wolfe-Simon, a young postdoctoral researcher who had spearheaded the project, stated that they had “cracked open the door to what's possible for life elsewhere in the universe - and that's profound". A senior NASA scientist claimed that their results would "fundamentally change how we define life", and, in attempting to convey the importance of the discovery to a reporter from the newspaper USA Today, referred to an episode from the original Star Trek television series in which the crew of the Starship Enterprise encounters a race of beings whose biochemistry is based on silica rather than carbon.
The new strain of bacteria, dubbed 'GFAJ-1', had been isolated from the arsenic-rich mud surrounding salty, alkaline Mono Lake in central California. What made the strain so unique, according to the NASA team, was that it had evolved the ability to substitute arsenate for phosphate in its DNA. Students of biology and chemistry know that phosphorus is one of the six elements that are absolutely required for life as we know it, and that DNA is a polymer linked by phosphate groups. Arsenic, which is directly below phosphorus on the periodic table, is able to assume a bonding arrangement like that of phosphate, so it might seem reasonable to wonder whether arsenate could replace phosphate in DNA and other biological molecules. Actually finding a living thing with arsenate-linked DNA would indeed be a momentous achievement in biology, as this would represent a whole new chemistry for the most fundamental molecule of life, and would change our understanding of the chemical requirements for life to exist on earth - and potentially other planets.
In 1987, Professor F.H. Westheimer of Harvard University published what would become a widely read commentary in Science Magazine entitled “Why Nature Chose Phosphates”. In it, he discussed the chemical properties that make the phosphate group so ideal for the many roles that it plays in biochemistry, chief among them the role of a linker group for DNA polymers. One of the critical characteristics of phosphate that Westheimer pointed out was that the bonds linking phosphate to organic molecules are stable in water. Clearly, if you are selecting a functional group to link your DNA, you don't want to choose one that will rapidly break apart in water. Among the functional groups that Westheimer compared to phosphate in terms of its suitability as a potential DNA linker was arsenate –but he very quickly dismissed the idea of arsenate-linked DNA because it would be far too unstable in water.

Mono Lake, California. (photo credit https://www.flickr.com/photos/slolane/)
Given this background, it is not hard to imagine that many scientists were puzzled, to say the least, by the NASA results. While the popular media took the announcement at face value and excitedly reported the results as a monumental discovery – NASA is, after all, a highly respected scientific body and the study was being published in Science Magazine, one of the most prestigious scientific journals in the world – many scientists quickly voiced their skepticism, mainly in the relatively new and unconstrained venue of the blogosphere. Microbiologist Rosie Redfield of the University of British Columbia, writing in her blog devoted to 'open science', wrote a detailed and highly critical analysis of the study. She pointed out, among other things, that the experimenters had failed to perform the critical purification and mass spectrometry analyses needed to demonstrate that arsenate was indeed being incorporated into the DNA backbone, and that the broth in which the bacteria were being grown actually contained enough phosphate for them to live and replicate using normal phosphate-linked DNA. Science journalist Carl Zimmer, in a column in the online magazine Slate, contacted twelve experts to get their opinions, and they were overwhelmingly negative. One of the experts said bluntly, “This paper should not have been published". Basically, the NASA researchers were making an astounding claim that, if true, would refute decades of established knowledge about the chemistry of DNA – but the evidence they presented was far from convincing. Carl Sagan's widely quoted dictum - “extraordinary claims require extraordinary evidence” - seemed to apply remarkably well to the situation.
What followed was a very public, very lively, and not always completely collegial debate among scientists about the proper way to discuss science: the NASA researchers appeared to dismiss the criticism amassed against their study because it came from blogs, websites, and Twitter feeds. The proper venue for such discussion, they claimed, was in the peer-reviewed literature. Critics countered that their refusal to respond to anything outside of the traditional peer-review system was disingenuous, because they had made full use of the publicity-generating power of the internet and mainstream media in the first place when they announced their results with such fanfare.
The traditional venue for debate, while quite a bit slower than the blogosphere, did eventually come through. When the full paper was published in Science a few months later, it was accompanied by eight 'technical comments' from other researchers pointing out deficiencies in the study, an 'editors note', and a broader news article about the controversy. In July of 2012, a paper was published in Science under the title “GFAJ-1 Is an Arsenate-Resistant, Phosphate-Dependent Organism”. The paper reported definitive evidence that DNA from GFAJ-1, under the conditions described in the NASA paper, did not have arsenate incorporated into its structure. Just like professor Westheimer discussed in the 1980s, it appears that nature really did choose phosphate – and only phosphate – after all . . . at least on this planet.
Background reading and viewing:
- Youtube video of the NASA press conference: http://www.youtube.com/watch?v=WVuhBt03z8g.
- Wolfe-Simon, F. et al. Science Express, Dec 2, 2010. The first preview article on the proposed 'arsenic bacteria'.
- Wolfe-Simon, F. et al., Science 2011, 332, 1163. The full research paper in Science Magazine.
- Westheimer, F.H. Science 1987, 235, 1173. The article by Westheimer titled 'Why Nature Chose Phosphates'.
- Zimmer, Carl, Slate, Dec 7, 2010: Blog post by Carl Zimmer titled 'This Paper Should Not Have Been Published'. http://www.slate.com/articles/health...published.html
- Redfield, R. Blog post Dec 4, 2010: http://rrresearch.fieldofscience.com...ria-nasas.html
- Science 2012, 337, 467. The paper in Science Magazine refuting the validity of the arsenic bacteria claim.




