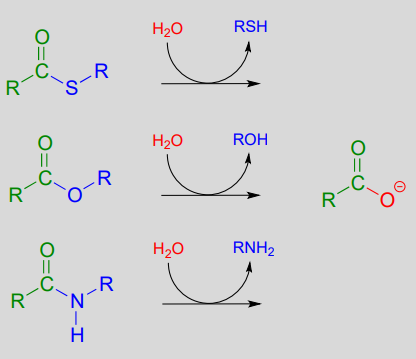
So far we have been looking at the formation of thioesters, carboxylic esters, and amides, starting from carboxylates. In hydrolytic acyl substitution reactions, nucleophilic water is the incoming nucleophile and a carboxylate group is the final product. Because carboxylates are the least reactive among the carboxylic acid derivatives, these hydrolysis reactions are thermodynamically favorable, with thioester hydrolysis the most favorable of the three.
Thioester, carboxylic ester, and amide hydrolysis:
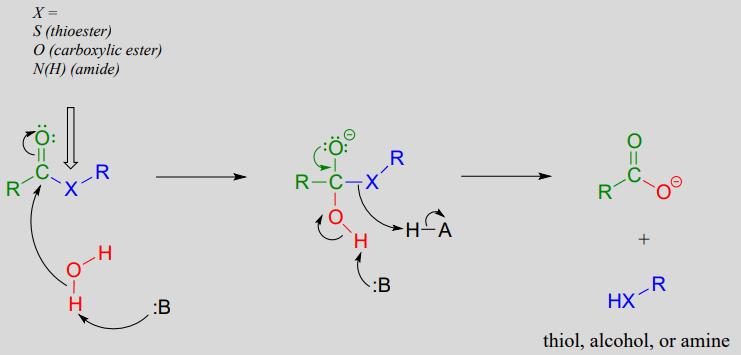
Mechanism:
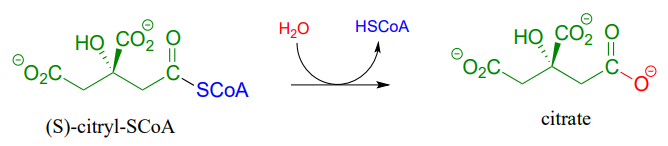
In the citric acid (Krebs) cycle, (S)-citryl CoA is hydrolyzed to citrate (EC 2.3.3.8):
Acetylcholinesterase (EC 3.1.1.7), an enzyme present in the synapse, catalyzes hydrolysis of the ester group in acetylcholine, a neurotransmitter that triggers muscle contraction.
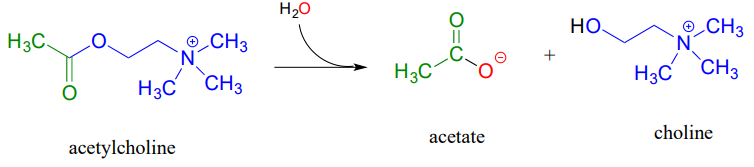
Like many other hydrolytic enzymes, the acetylcholinesterase reaction proceeds in two phases: first, a covalent enzyme-substrate intermediate is formed when the acyl group of acetylcholine is transferred to an active-site serine on the enzyme (a transesterification reaction). A water nucleophile then attacks this ester, driving off acetate and completing the hydrolysis.
Based on the above description, draw the structure of the covalent enzyme-substrate intermediate in the acetylcholinesterase reaction.
If the action of acetylcholinesterase is inhibited, acetylcholine in the synapse does not get hydrolyzed and thus accumulates, resulting in paralysis and death in severe cases. Sarin nerve gas is a potent inhibitor of acetylcholinasterase action. Some victims of the Tokyo subway sarin attack in 1995 who were exposed to low levels of the gas reported that they initially realized that something was wrong when they noticed how dark everything seemed around them. This was due to uncontrolled contraction of their pupils. You will be invited to consider the mechanism of inhibition by sarin in problem 11.6.4.
Peptide (amide) bonds in proteins and polypeptides are subject to spontaneous (nonenzymatic) hydrolysis in water.
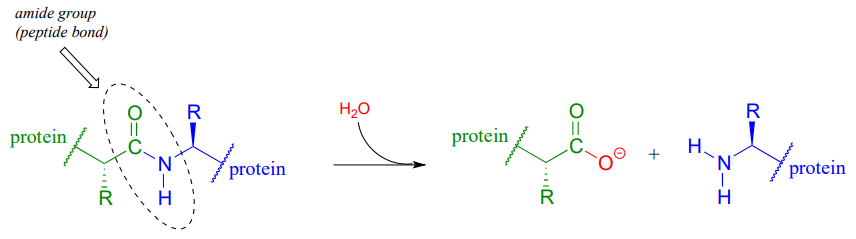
Although this amide to carboxylate conversion is thermodynamically a downhill reaction, peptide bonds are kinetically very stable (they react slowly) at neutral pH. In fact, the half-life for uncatalyzed hydrolysis of a peptide bond in pH 7 water is by some estimates as high as 1000 years. (Ann. Rev. Biochem. 2011, 80, 645.)
The stability of peptides bonds makes good physiological sense: we would all be in trouble if our enzymes, receptors, and structural proteins were hydrolyzing away while we slept! That being said, it is also true that controlled, specific hydrolysis of peptide bonds, catalyzed by a large, diverse class of enzymes called proteases, is a critical biochemical reaction type that can occur very rapidly, in many different biological contexts. For example, many proteins only become active after they have been ‘processed' - in other words, hydrolyzed at a specific amino acid location by a specific protease.
Although all proteases catalyze essentially the same reaction – amide hydrolysis - different protease subfamilies have evolved different catalytic strategies to accomplish the same result. HIV protease is the target of some the most recently-developed anti-HIV drugs. It plays a critical role in the life cycle if the HIV virus, hydrolyzing specific peptide bonds of essential viral proteins in order to convert them to their active forms. HIV protease is a member of the aspartyl protease subfamily, so-named because of the two aspartate residues located in the active sites of these enzymes. HIV protease is also, as you are probably aware, the target of HIV protease inhibitor drugs, which are a component of the most effective treatment currently available for HIV infection.
In HIV protease and other aspartyl proteases, the two enzymatic aspartates residues (shaded grey and abbreviated 'Asp1' and 'Asp2 'in the figure below) work in concert to activate the electrophile, nucleophile, and leaving group in the reaction.
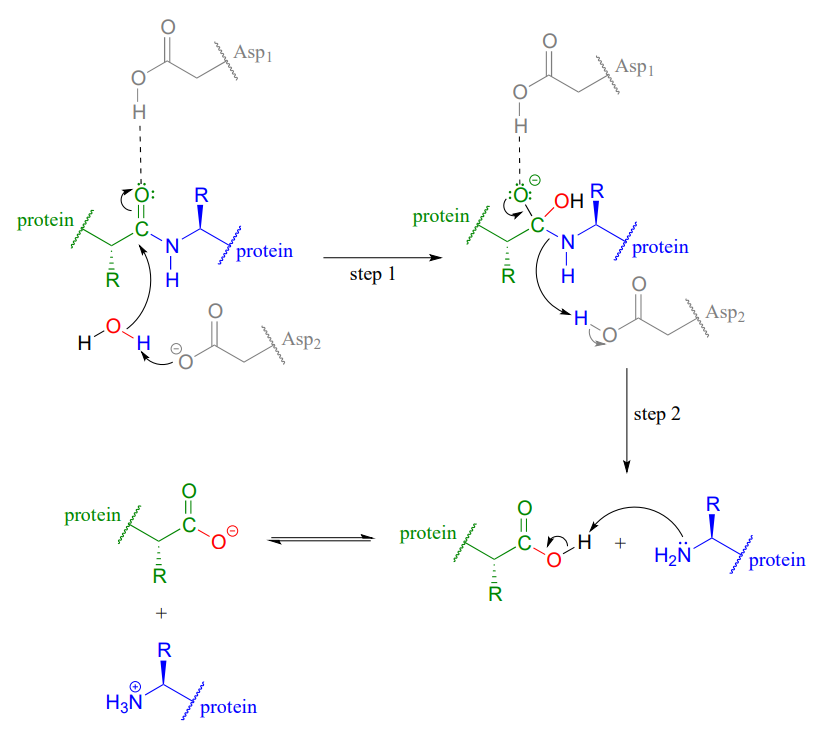
Exactly how this works is a subject of some debate and the details may well vary according to the enzyme in question, but one likely mechanism is illustrated in the figure above, where Asp1, which is initially in its protonated form, contributes a hydrogen bond to draw electron density away from the carbonyl carbon, making it more electrophilic. At the same time, Asp2, which begins the reaction cycle in its anionic form, deprotonates the water molecule to make it more nucelophilic. In step 2, Asp2 donates a proton back to the nitrogen, making it a better leaving group.
HIV protease inhibitors shut down this reaction, which prevents the virus from processing the proteins that it uses to bond to host cells.
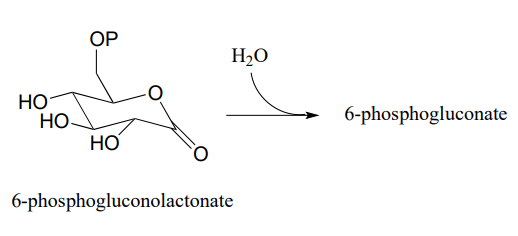
Lactonase (EC 3.1.1.17), the second enzyme in the oxidative branch of the pentose phosphate pathway, catalyzes hydrolysis of the lactone (cyclic ester) group in 6-phosphogluconolactone. Draw the structure of 6-phosphogluconate, the product of this reaction.
Draw the product of the \(\beta \)-lactamase-catalyzed hydrolysis of penicillin as described in section 11.6.
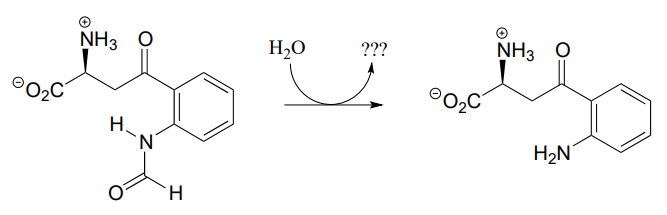
What is the missing product (designated below by question marks) in the reaction below, which is part of degradation pathway for the amino acid tryptophan? How could you describe this reaction in organic chemistry terminology?
- Answer
-
Add answer text here and it will automatically be hidden if you have a "AutoNum" template active on the page.










