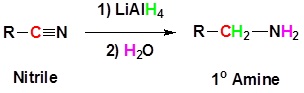Preparation of Primary Amines
Although direct alkylation of ammonia by alkyl halides leads to 1º-amines, alternative procedures are preferred in many cases. These methods require two steps, but they provide pure product, usually in good yield. The general strategy is to first form a carbon-nitrogen bond by reacting a nitrogen nucleophile with a carbon electrophile. The following table lists several general examples of this strategy in the rough order of decreasing nucleophilicity of the nitrogen reagent. In the second step, extraneous nitrogen substituents that may have facilitated this bonding are removed to give the amine product.
|
Nitrogen
Reactant
|
Carbon
Reactant
|
1st Reaction
Type
|
Initial Product
|
2nd Reaction
Conditions
|
2nd Reaction
Type
|
Final Product
|
| N3(–) |
RCH2-X or
R2CH-X |
SN2 |
RCH2-N3 or
R2CH-N3 |
LiAlH4 or
4 H2 & Pd |
Hydrogenolysis |
RCH2-NH2 or
R2CH-NH2 |
| C6H5C2O2NH / OH- |
RCH2-X or
R2CH-X |
SN2 |
RCH2-NC2O2C6H5 or
R2CH-NC2O2C6H5 |
NaOH / heat |
Hydrogenolysis |
RCH2-NH2 or
R2CH-NH2 |
| CN(–) |
RCH2-X or
R2CH-X |
SN2 |
RCH2-CN or
R2CH-CN |
LiAlH4 |
Reduction |
RCH2-CH2NH2 or
R2CH-CH2NH2 |
| NH3 |
RCH=O or
R2C=O |
Addition /
Elimination |
RCH=NH or
R2C=NH |
H2 & Ni
or NaBH3CN |
Reduction |
RCH2-NH2 or
R2CH-NH2 |
| NH3 |
RCOX |
Addition /
Elimination |
RCO-NH2 |
LiAlH4 |
Reduction |
RCH2-NH2 |
NH2CONH2
(urea) |
R3C(+) |
SN1 |
R3C-NHCONH2 |
NaOH soln. |
Hydrolysis |
R3C-NH2 |
A specific example of each general class is provided in the diagram below. In the first two, an anionic nitrogen species undergoes an SN2 reaction with a modestly electrophilic alkyl halide reactant. For example #2, the Gabriel synthesis is shown. The alkaline conditions deprotonate phthalmide to create a strong nucleophile for SN2 reactions with alkyl halides. Example #3 also starts with an SN2 reaction of cyanide with an alkyl halide following by reduction of the cyano group to form a primary amine that extends the carbon system of the alkyl halide by a methylene group (CH2). In all three of these methods 3º-alkyl halides cannot be used because the major reaction path is an E2 elimination.
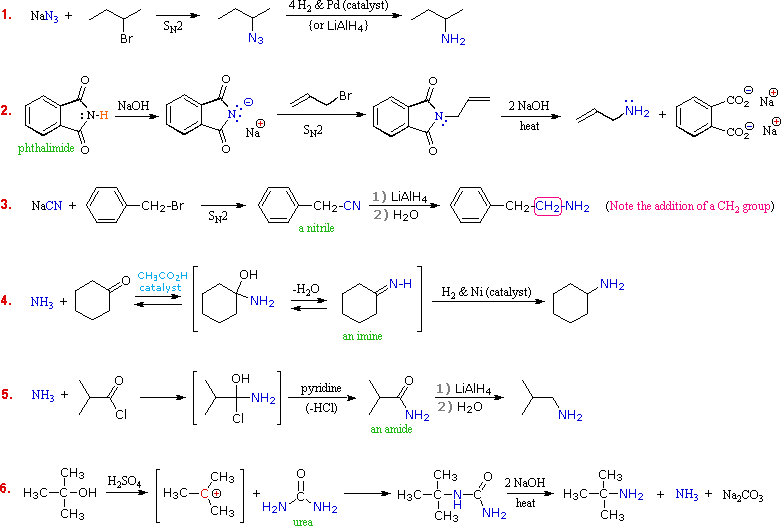
The methods illustrated by examples #4 and #5 proceed by the reaction of ammonia, or equivalent nitrogen nucleophiles, with the electrophilic carbon of a carbonyl group. A full discussion of carbonyl chemistry is presented in an independent chapter, but for present purposes it is sufficient to recognize that the C=O double bond is polarized so that the carbon atom is electrophilic. Nucleophilic addition to aldehydes and ketones is often catalyzed by acids. Acid halides and anhydrides are even more electrophilic, and do not normally require catalysts to react with nucleophiles. The reaction of ammonia with aldehydes or ketones occurs by a reversible addition-elimination pathway to give imines (compounds having a C=N function). These intermediates are not usually isolated, but are reduced as they are formed (i.e. in situ). Acid chlorides react with ammonia to give amides, also by an addition-elimination path, and these are reduced to amines by LiAlH4.
The 6th example is a specialized procedure for bonding an amino group to a 3º-alkyl group (none of the previous methods accomplishes this). Since a carbocation is the electrophilic species, rather poorly nucleophilic nitrogen reactants can be used. Urea, the diamide of carbonic acid, fits this requirement nicely. The resulting 3º-alkyl-substituted urea is then hydrolyzed to give the amine. One important method of preparing 1º-amines, especially aryl amines, uses a reverse strategy. Here a strongly electrophilic nitrogen species (NO2(+)) bonds to a nucleophilic carbon compound. This nitration reaction gives a nitro group that can be reduced to a 1º-amine by any of several reduction procedures.
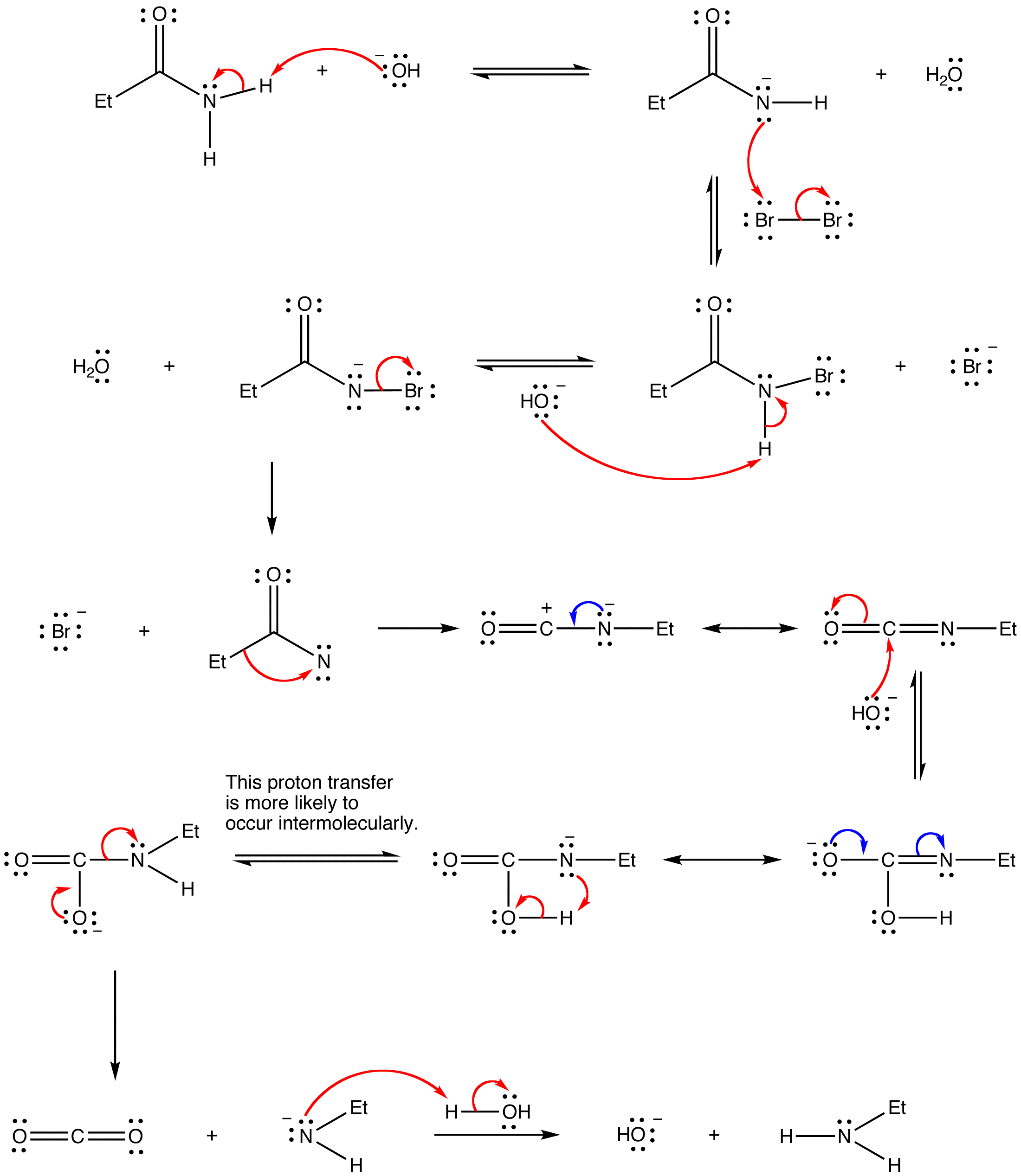
Hofmann rearrangement
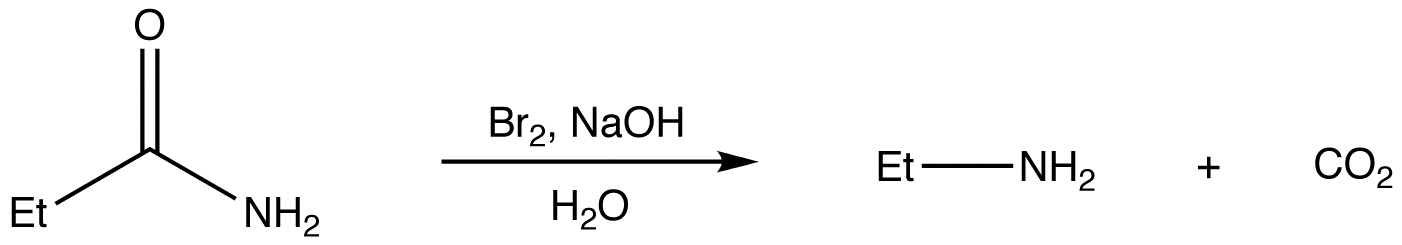
Hofmann rearrangement, also known as Hofmann degradation and not to be confused with Hofmann elimination, is the reaction of a primary amide with a halogen (chlorine or bromine) in strongly basic (sodium or potassium hydroxide) aqueous medium, which converts the amide to a primary amine. For example:
Mechanism:
Curtius Rearrangement
The Curtius rearrangement involves an acyl azide.

The mechanism of the Curtius rearrangement involves the migration of an -R group form the carbonyl carbon to the the neighboring nitrogen.

Reduction of Nitro Groups
Several methods for reducing nitro groups to amines are known. These include catalytic hydrogenation (H2 + catalyst), zinc or tin in dilute mineral acid, and sodium sulfide in ammonium hydroxide solution. The procedures described above are sufficient for most case

Reduction of Nitriles
Nitriles can be converted to primary amines by reaction with lithium aluminum hydride. During this reaction the hydride nucleophile reacts with the electrophilic carbon in the nitrile to form an imine anion. Once stabilized by a Lewis acid-base complexation the imine salt can accept a second hydride to form a dianion. The dianion can then be converted to an amine by addition of water.