General Review
20-1 Predict which amine is more basic and provide a reason for your answer.
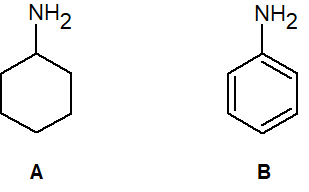
20-2 Give the product of the following reaction.
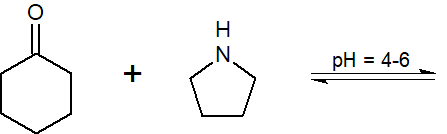
20-3 Predict the final product of the following reaction chain and give its IUPAC name.
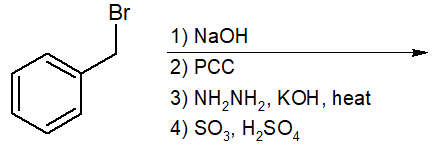
20-4 Propose a route of synthesis from nitrobenzene to the given product. Assume the given molecule is the major product, and for the purposes of this problem, ignore its isomers.
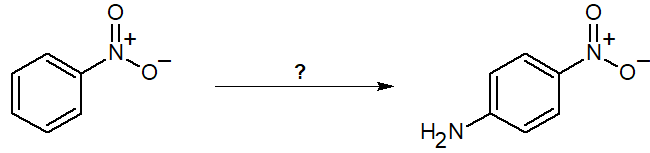
20-5 Choose the correct answer that describes the product of the following Cope elimination reaction.

a) N,N,2-trimethylpropan-1-iminium
b) N,N,2-trimethylpropan-1-amine
c) 2-methylprop-1-ene and N-hydroxy-N-methylmethanamine
d) N-hydroxy-N,2-dimethylpropan-1-amine
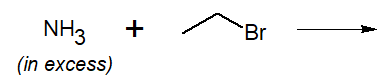
20-6 Explain why the following reaction might not be the best way to synthesize ethanamine.
Basicity and Effects of Amines
20-7 Draw all possible resonance structures for aniline and cyclohexanamine.

20-8 Identify which of the following nitroaniline isomers is the most basic and give a reason for your answer.

20-9 For the following compounds, identify which substituents are pi-acceptors of the electrons from the amine group (if applicable) and if they are, draw their resonance structure to show the movement of electrons.
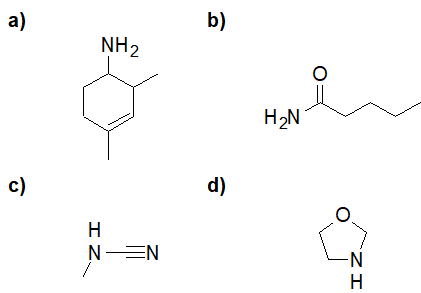
Aromatic Substitution of Arylamines and Pyridin
20-10 Explain why the following arylamine needs to be turned into an amide before a Friedel-Crafts acylation and then predict the final product of the reaction.
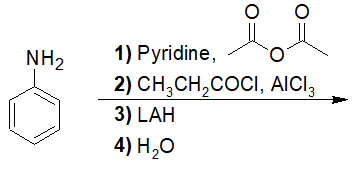
20-11 Predict the products of the following reactions.
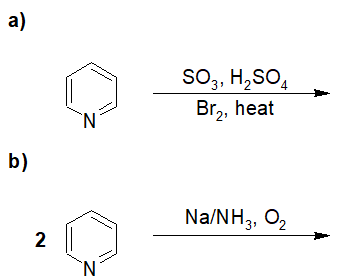
20-12 Predict the product of the following reaction and provide the correct IUPAC nomenclature.
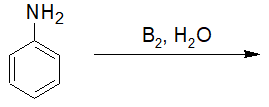
Alkylation and Acylation of Amines
20-13 Predict the product of the following acylation reaction.
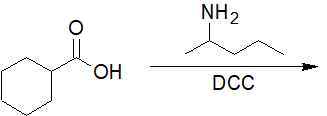
20-14 Suggest a route of synthesis for the following compound, starting with benzoyl chloride.

20-15 Choose the correct product of the following reaction.
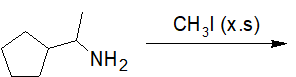
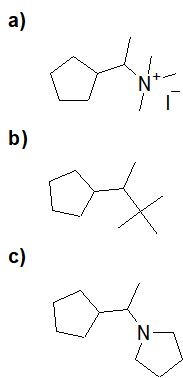
Formation of Sulfonamides
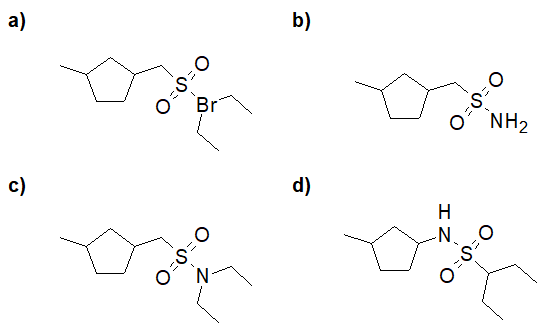
20-16 Choose the correct structure of the product of the following reaction.
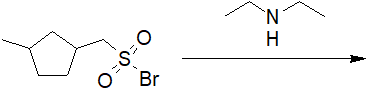
20-17 Provide the structure of the intermediate compound and final product of the following reaction.
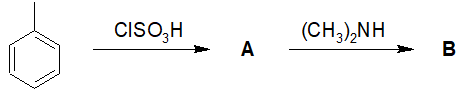
20-18 Predict the product of the following reaction.
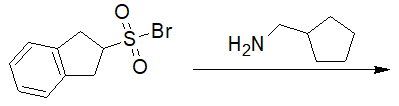
Amines as Leaving Groups: The Hofmann Elimination
20-19 Predict the major alkene product of the following Hofmann elimination reaction and give the proper IUPAC nomenclature.
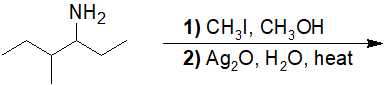
20-20 Choose the correct product of the following reaction.
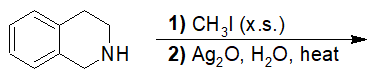
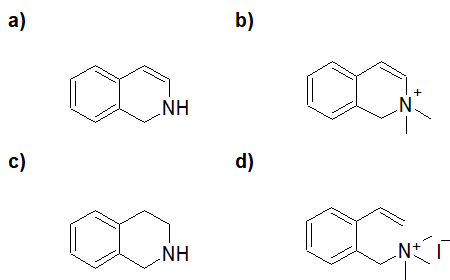
20-21 Propose a route of synthesis from pentan-1-amine to pentanal (include a Hofmann elimination reaction).

Oxidation of Amines: The Cope Elimination
20-22 Predict the structure and give the proper IUPAC nomenclature of the product of the following reaction.

20-23 Propose a route of synthesis for the following compound, starting with cyclohexanecarboxylic acid and include a Cope elimination reaction.
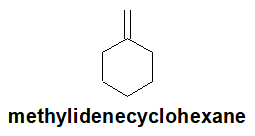
20-24 Predict the structure of the product of the following reaction.
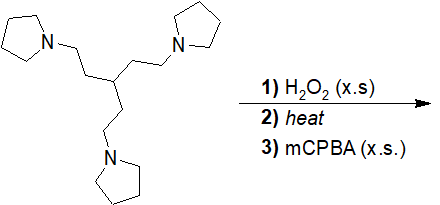
Reactions of Amines with Nitrous Acid
20-25 Predict the product of the following reaction and provide the correct IUPAC nomenclature.

20-26 Predict the products of the following reactions.

20-27 Suggest a route of synthesis for the following product, starting with aniline.
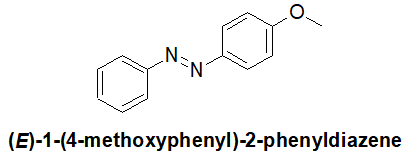
Synthesis of Amines by Reductive Amination and Acylation-Reduction
20-28 Predict the product of the following reaction and provide its IUPAC nomenclature.
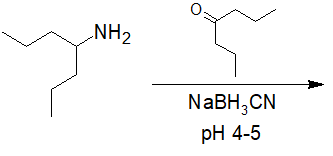
20-29 Identify which route of synthesis is the better way to make N-(cyclopentylmethyl)- -N-methylethanamine and then show the intermediate molecules for the correct path.
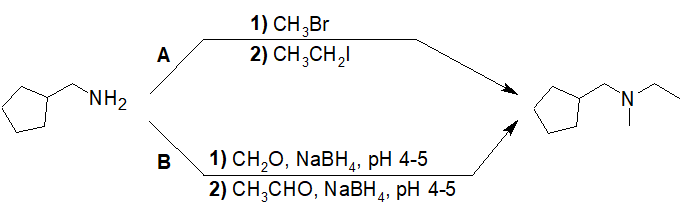
20-30 Choose the correct IUPAC nomenclature of the product of the following reaction.
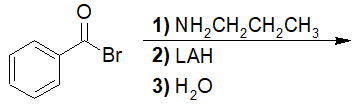
a) N-propylbenzamide
b) phenyl(propylamino)methanol
c) N-benzylpropan-1-amine
d) benzamide

