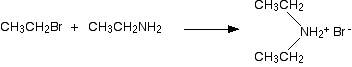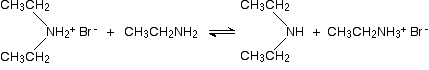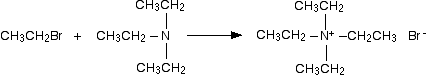Amines as Nucleophiles
Amines seldom serve as leaving groups in nucleophilic substitution or base-catalyzed elimination reactions. Indeed, they are even less effective in this role than are hydroxyl and alkoxyl groups. While we will see another section that it is possible to coax the amine to serve as a leaving group. As weak bases, amines are good nucleophiles.
Amines and Carbonyls
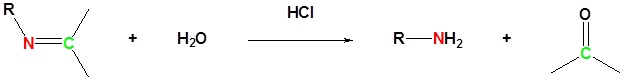
The reaction of aldehydes and ketones with ammonia or 1º-amines forms imine derivatives, also known as Schiff bases (compounds having a C=N function). Water is eliminated in the reaction, which is acid-catalyzed and reversible in the same sense as acetal formation. The pH for reactions which form imine compounds must be carefully controlled. The rate at which these imine compounds are formed is generally greatest near a pH of 5, and drops at higher and lower pH's. At high pH there will not be enough acid to protonate the OH in the intermediate to allow for removal as H2O. At low pH most of the amine reactant will be tied up as its ammonium conjugate acid and will become non-nucleophilic.
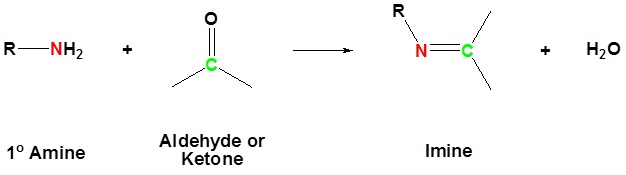
Imine formation is reversible. Imines can be hydrolyzed back to the corresponding primary amine under acidic aqueous conditions.
Most aldehydes and ketones react with 2º-amines to give products known as enamines.
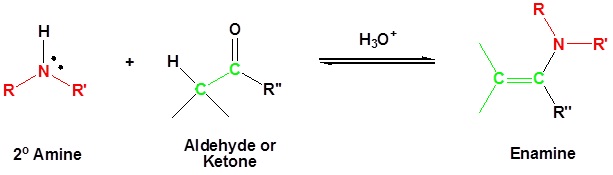
It should be noted that, like acetal and imine formation, these are acid-catalyzed reversible reactions in which water is lost. Consequently, enamines are easily converted back to their carbonyl precursors by acid-catalyzed hydrolysis.

Amines and Acid Chlorides
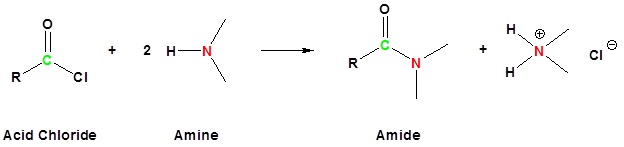
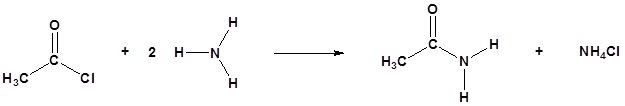
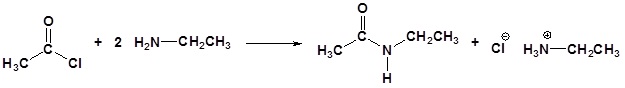
Acid chlorides react with ammonia, 1o amines and 2o amines to form amides.
Amines and Sulfonyl Chloride
The sulfonyl group is the sulfur-analog to the carbonyl group. Both groups contain an electrophilic carbonyl carbon with chloride as an excellent leaving group. Because sulfur is a third shell element it can form "expanded octets".
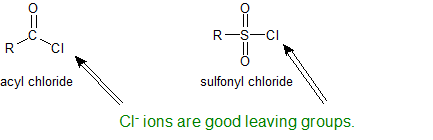
Amines react with sulfonyl groups to form sulfonamides. Sulfonamides are used as antimicrobial agents therapeutically and called sulfa drugs. The reaction to form sulfonamides occurs under alkaline conditions to keep the amine nucleophilic. Any time amines are present in an aqueous solution, measurable hydroxide is present. The mechanism for the sulfonation reaction is analogous to the acylation mechanism as is shown below.
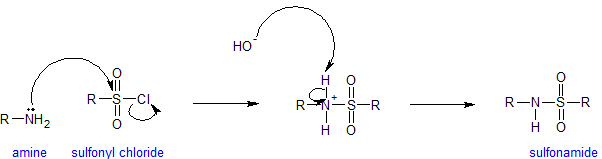
The end of this chapter includes some additional information on sulfonamides.
Alkylations
It is instructive to examine nitrogen substitution reactions using common alkyl halides as the electrophiles. Thus, reaction of a primary alkyl bromide with a large excess of ammonia yields the corresponding 1º-amine, presumably by an SN2 mechanism. The hydrogen bromide produced in the reaction combines with some of the excess ammonia, giving ammonium bromide as a by-product. Water does not normally react with 1º-alkyl halides to give alcohols, so the enhanced nucleophilicity of nitrogen relative to oxygen is clearly demonstrated.
2 RCH2Br + NH3 (large excess)  RCH2NH2 + NH4(+) Br(–) RCH2NH2 + NH4(+) Br(–) |
It follows that simple amines should also be more nucleophilic than their alcohol or ether equivalents. If, for example, we wish to carry out an SN2 reaction of an alcohol with an alkyl halide to produce an ether (the Williamson synthesis), it is necessary to convert the weakly nucleophilic alcohol to its more nucleophilic conjugate base for the reaction to occur. In contrast, amines react with alkyl halides directly to give N-alkylated products. Since this reaction produces HBr as a co-product, hydrobromide salts of the alkylated amine or unreacted starting amine (in equilibrium) will also be formed.
2 RNH2 + C2H5Br  RNHC2H5 + RNH3(+) Br(–) RNHC2H5 + RNH3(+) Br(–)  RNH2C2H5(+) Br(–) + RNH2 RNH2C2H5(+) Br(–) + RNH2 |
Unfortunately, the direct alkylation of 1º or 2º-amines to give a more substituted product does not proceed cleanly. If a 1:1 ratio of amine to alkyl halide is used, only 50% of the amine will react because the remaining amine will be tied up as an ammonium halide salt (remember that one equivalent of the strong acid HX is produced). If a 2:1 ratio of amine to alkylating agent is used, as in the above equation, the HX issue is solved, but another problem arises. Both the starting amine and the product amine are nucleophiles. Consequently, once the reaction has started, the product amine competes with the starting material in the later stages of alkylation, and some higher alkylated products are also formed. Even 3º-amines may be alkylated to form quaternary (4º) ammonium salts. When tetraalkyl ammonium salts are desired, as shown in the following example, Hünig's base may be used to scavenge the HI produced in the three SN2 reactions. Steric hindrance prevents this 3º-amine (Hünig's base) from being methylated.
C6H5NH2 + 3 CH3I + Hünig's base  C6H5N(CH3)3(+) I(–) + HI salt of Hünig's base
C6H5N(CH3)3(+) I(–) + HI salt of Hünig's base
You get a complicated series of reactions on heating primary amines with halogenoalkanes to give a mixture of products - probably one of the most confusing sets of reactions you will meet at this level. The products of the reactions include secondary and tertiary amines and their salts, and quaternary ammonium salts.
Making secondary amines and their salts
In the first stage of the reaction, you get the salt of a secondary amine formed. For example if you started with ethylamine and bromoethane, you would get diethylammonium bromide
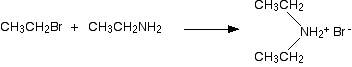
In the presence of excess ethylamine in the mixture, there is the possibility of a reversible reaction. The ethylamine removes a hydrogen from the diethylammonium ion to give free diethylamine - a secondary amine.
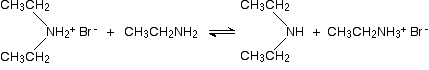
Making tertiary amines and their salts
But it doesn't stop here! The diethylamine also reacts with bromoethane - in the same two stages as before. This is where the reaction would start if you reacted a secondary amine with a halogenoalkane.
In the first stage, you get triethylammonium bromide.

There is again the possibility of a reversible reaction between this salt and excess ethylamine in the mixture.
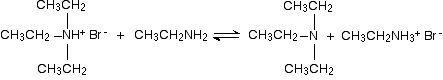
The ethylamine removes a hydrogen ion from the triethylammonium ion to leave a tertiary amine - triethylamine.
Making a quaternary ammonium salt
The final stage! The triethylamine reacts with bromoethane to give tetraethylammonium bromide - a quaternary ammonium salt (one in which all four hydrogens have been replaced by alkyl groups).
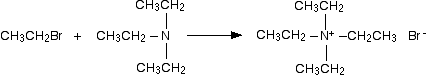
This time there isn't any hydrogen left on the nitrogen to be removed. The reaction stops here.
Exercise
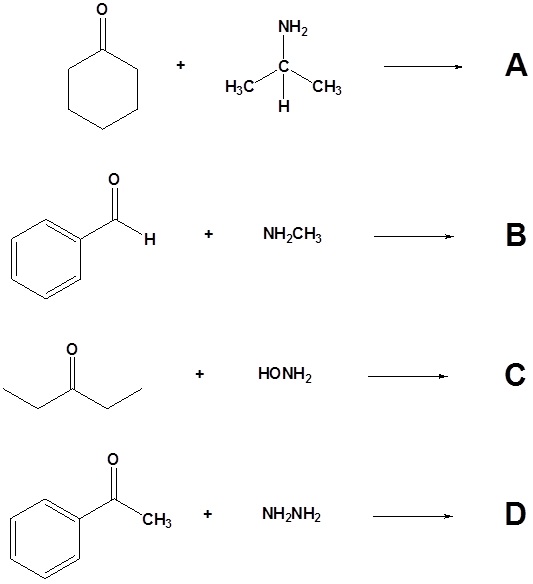
10. Draw the products of the following reactions.
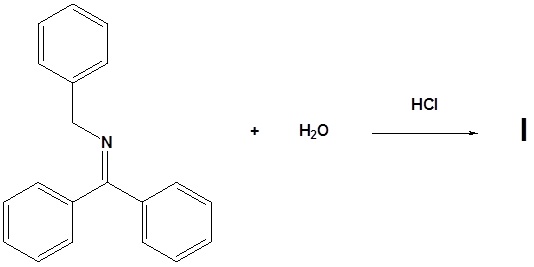
11. Draw the structure of the reactant needed to produce the indicated product.
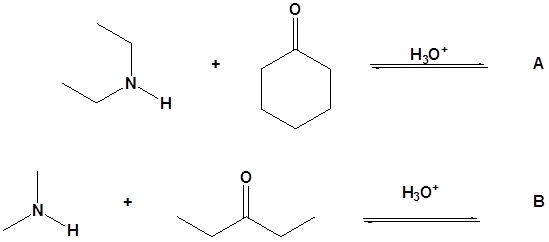
12. Draw the products for the following reactions.
13. Draw the missing reactant to complete each reaction below.
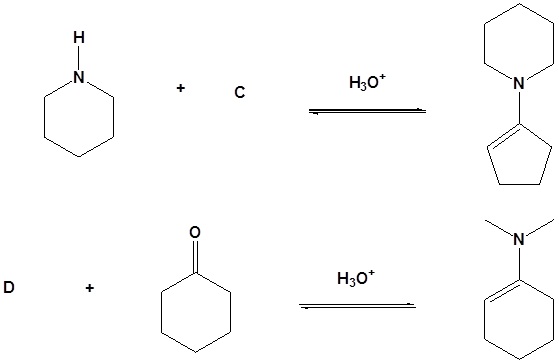
Answer
-
10.
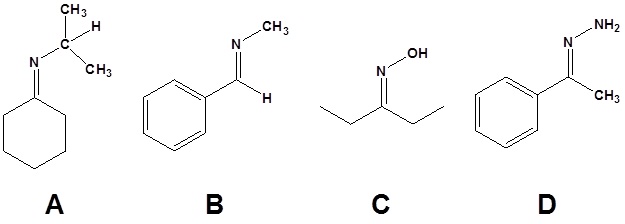
11.
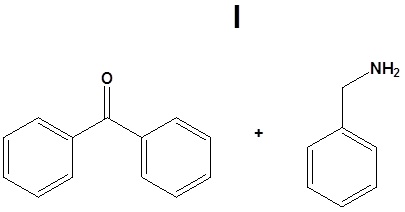
12.
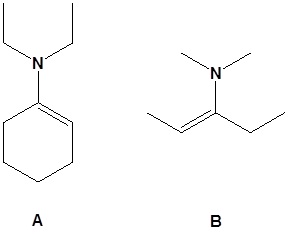
13.
Contributors and Attributions








 RCH2NH2 + NH4(+) Br(–)
RCH2NH2 + NH4(+) Br(–) RNH2C2H5(+) Br(–) + RNH2
RNH2C2H5(+) Br(–) + RNH2