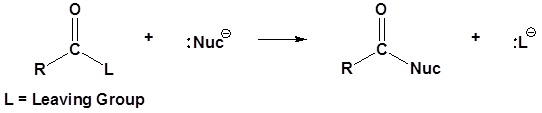
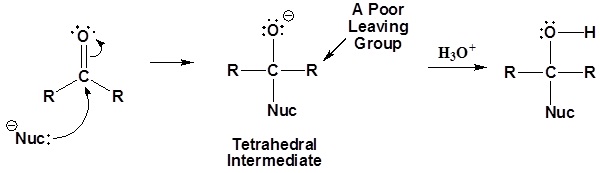
General reaction
Carboxylic acid derivatives are electrophilic and can react with nucleophiles to form nucleophilic acyl substitution products. The driving force of these reactions is the stability of the leaving group shown as :L- below.
General mechanism
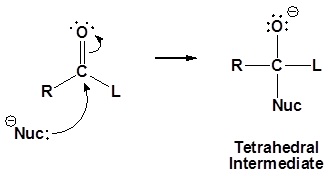
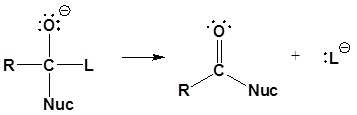
The nucleophile reacts with the electrophilic carbonyl carbon to form the tetrahedral intermediate. When the carbonyl reforms, the leaving group is lost to form the substitution product as shown in the mechanism below.
1) Nucleophilic reaction at the carbonyl
2) Carbonyl reforms and leaving group is removed
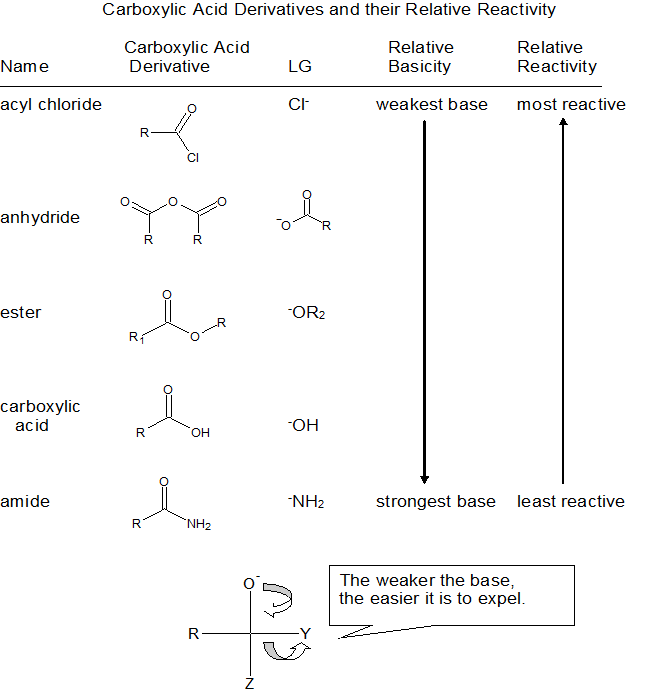
Relative Reactivity to Nucleophilic Acyl Substitution
The relative reactivity of carbonyl compounds toward nucleophile substitutions is related to the stability of the leaving group - the more stable the leaving group, the more favorable the substitution reaction. Evaluating leaving group stability is analogous to evaluating conjugate base stability as shown in the table below.
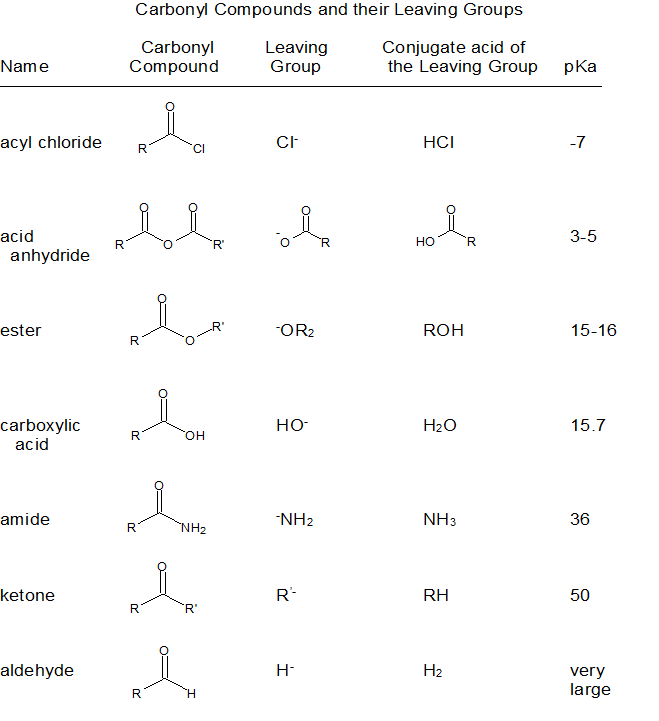
Although aldehydes and ketones also contain carbonyls, their chemistry is distinctly different because they do not contain suitable leaving groups. Once a tetrahedral intermediate is formed, aldehydes and ketones cannot reform their carbonyls because the carbide (RC-) and hydride (H-) leaving groups are too unstable. Therefore, aldehydes and ketones typically undergo nucleophilic additions and not substitutions.
Integrating all of this information into the single table below summarizes the relative reactivity of carboxylic acids and their derivatives.
Acid Derivative Interconversion
From this understanding, multiple step synthesis strategies can be developed. The derivatives with the most stable leaving groups can be used to synthesize the derivatives with the least stable leaving groups as illustrated in the diagram below.
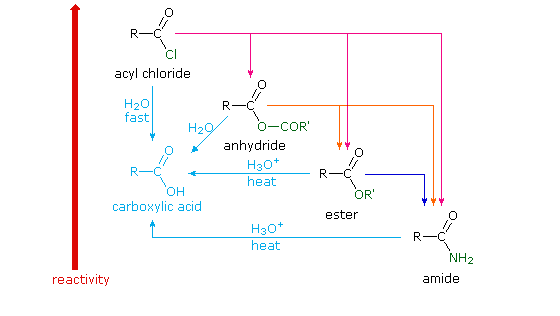
While it may appear from diagram above that the acyl chlorides are the "source" of the acid derivatives, acyl chlorides are so highly reactive that it is common to convert the carboxylic acid to the acid chloride and then immediately form the derivative. From a laboratory synthesis perspective, the reaction sequence begins with the carboxylic acid.
Exercise
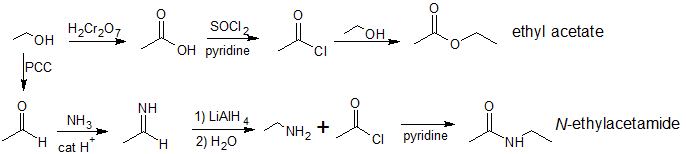
3. Using ethanol as the only source of carbons in the final products, show how to synthesize ethyl acetate and N-ethylacetamide.
- Answer
-
3.
Contributors and Attributions









