Learning Objective
- apply bonding theories to the structure of alkynes and distinguish between internal and terminal triple bonds
Alkynes: Terminal vs Internal
Alkynes are organic molecules with carbon-carbon triple bonds. They are unsaturated hydrocarbons with the empirical formula of CnH2n-2. The simplest alkyne is ethyne which has the common name acetylene. Acetylene is a common name to memorize.
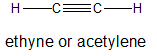
It is important to distinguish between terminal and internal alkynes because they can undergo different patterns of reactivity.
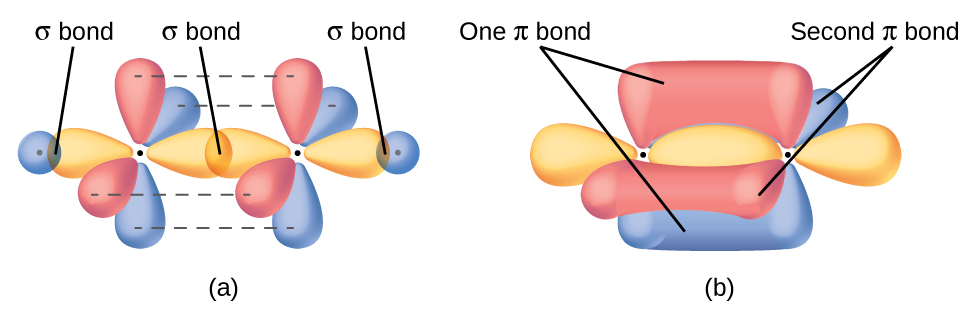
Electronic Structure
The sp hybridization of the carbon-carbon triple bond results in the perpendicular orientation of the sigma bond and two pi bonds. The close proximity of the electrons in this geometry orientation creates molecules with less stability. The structure of the carbon-carbon triple bond strongly influences the chemical reactivity of alkynes and the acidity of terminal alkynes. Because of its linear configuration (the bond angle of a sp-hybridized carbon is 180º), a ten-membered carbon ring is the smallest that can accommodate this function without excessive strain.
Physical Properties
Alkynes are nonpolar, unsaturated hydrocarbons with physical properties similar to alkanes and alkenes. Alkynes dissolve in organic solvents, have slight solubility in polar solvents, and are insoluble in water. Compared to alkanes and alkenes, alkynes have slightly higher boiling points. For example, ethane has a boiling point of -88.6 C, while ethene is -103.7 C and ethyne has a higher boiling point of -84.0 ?C.
Exercise
- Arrange ethane, ethene, and acetylene in order of decreasing carbon-carbon length.
- How many pi bonds and sigma bonds are involved in the structure of ethyne?
- What contribute to the weakness of the pi bonds in an alkyne?
- Arrange the following hydrocarbons in order of decreasing boiling point: 1-heptyne, 1-hexyne, 2-methyl-1-hexyne.
- Predict the solvent with greater 2-butyne solubility. a) water or 1-octanol? b) water or acetone? c) ethanol or hexane?
- Answer
-
1. relative carbon-carbon bond length: ethane < ethene < acetylene
2. There are three sigma bonds and two pi bonds.
3. The sigma bond and two pi bonds are all perpendicular to each other in the triple bond creating electron repulsion between the three pairs of bonding electrons in the triple bond.
4. 1-heptyne (99.7C) > 2-methyl-1-hexyne (91C) > 1-hexyne (71C)
5. a) 1-octanol b) acetone c) hexane
Outside links
- www.ucc.ie/academic/chem/dolc...t/alkynes.html
- www.cliffsnotes.com/WileyCDA/...eId-22631.html
References
- Bloch, D.R. Organic chemistry demystified, New York : McGraw-Hill, 2006.
- Vollhardt. Schore, Organic Chemistry Structure and Function Fifth Edition, New York: W.H. Freeman and Company, 2007.
Contributors and Attributions
- Bao Kha Nguyen, Garrett M. Chin