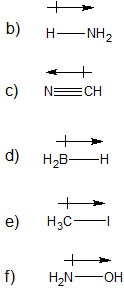1-1:
a) 6 v.e. / 2 covalent bonds
b) 4 v.e. / 4 covalent bonds
c) 7 v.e. / 1 covalent bond
d) 6 v.e. / 2 covalent bonds (can also expand the octet to make 6 covalent bonds)
e) 1 v.e. / 1 covalent bond
f) 3 v.e. / 3 covalent bonds
1-2:
(d) K +
1-3:
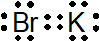
1-4:
(c) PCl
Lewis Structures
1-5:
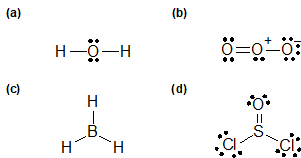
1-6:
a) Neon, 8 valence electrons
b) Magnesium, 2 valence electrons
c) Oxygen, 6 valence electrons
d) Bromine, 7 valence electrons
1-7:
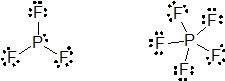
1-8:
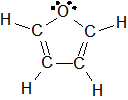
1-9: C.
Electronegativity and Bond Polarity
1-10:
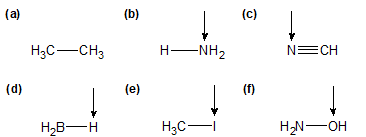
1-11:
a) no dipole moment arrow b/c non-polar
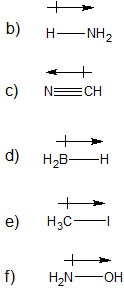
1-12:
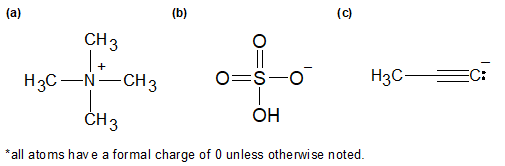
1-13:
a) 0
b) -1
c) -2
1-14:
a) 0
b) -1
c) 0
d) +1
Ionic Structures
1-15:
a) Na+ and Cl-
b) Mg+2 and 2 Br-
c) K+ and NO3-
d) Na+ and H2PO4-
1-16:
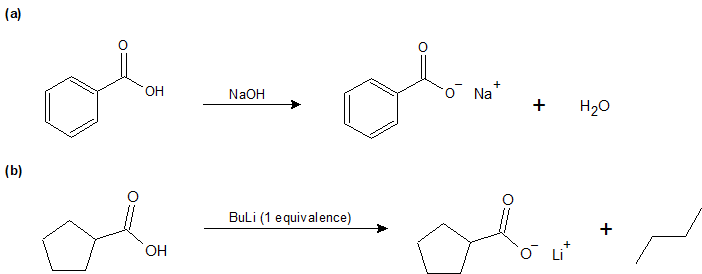
1-17:
a) sodium cyanide
b) CaC2O4
c) aluminum hydroxide
d) Sn3(PO4)2
e) KClO
Resonance
1-18:
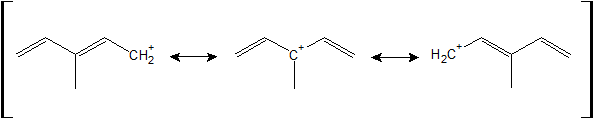
1-19:
The resonance structure that is most stable has the tertiary carbocation. This tertiary carbocation is stabilized by hyperconjugation as well as two possible directions for resonance (compared to one immediate resonance structure for the other two carbocations).
1-20:

1-21:
When comparing the deprotonated forms of acetic acid and ethanol, acetate and ethoxide respectively, you can observe that acetate delocalizes the negative charge over the entire carboxylate group. Ethoxide, however, can only hold the negative charge on the alkoxide, making it a better base, but worse as an acid.
1-22:
1-23:
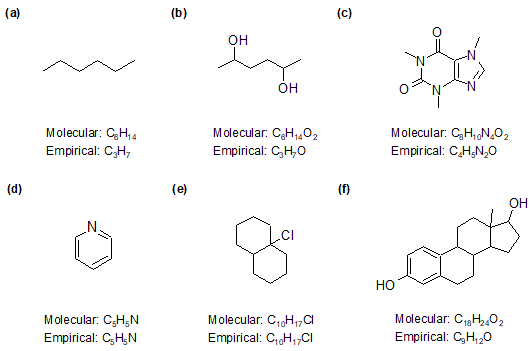
1-24:
1-25:
False; empirical formulas are the simplest whole number ratios that are useful in calculating percent compositions of atoms in a molecule. However, as they do not give the absolute number of atoms in a molecule, they cannot be used to calculate the molecular weight of the molecule.
1-26:
a) C2H2O
b) C4H3N
c) C9H7NO
Acids and Bases - Arrhenius, Bronsted-Lowry, and Lewis
1-27:
Arrhenius: An Arrhenius acid is a species that will donate a H+ when dissolved in water. An Arrhenius base is a species that will break down to yield a OH- when dissolved in water.
Bronsted-Lowry: A Bronsted-Lowry acid is a species that will donate a H+ when dissolved in solution (not only in water). A Bronsted-Lowry base is a species that can accept a H+ in solution (not only in water).
Lewis: A Lewis acid is an electron pair acceptor. A Lewis base is an electron base donor.
1-28:
Ka = 2.4 x 101
1-29:
H3O+ > HF > NH4+ > H2O
1-30:
NH2- > H2O > CH3COO- > HSO4-
1-31:
Compound A is the stronger base. Compound B is the stronger acid.
1-32:
Group A will want to grab the H+ more than group B. Since C is less electronegative than N, it cannot stabilize the negative charge as well and will want to grab a H+ in order to get rid of the charge. (grab = react with)