There is another reaction which occurs at the atom directly attached to an aromatic ring. This is known as "side chain oxidation." When a compound which has an alkyl group directly attached to an aryl group is treated with a strong oxidizing agent like potassium permanganate (KMnO4) or Jones Reagent (CrO3/H2SO4), the benzylic carbon is oxidized to a carboxylic acid group which remains attached to the aryl group. Any other carbon-carbon bonds in the alkyl group are broken. For the oxidation reaction, the number of carbon atoms in the alkyl side chain does not matter, However, the benzylic carbon must have at least one benzylic hydrogen attached. Thus, tertiary carbons attached to an aromatic ring are not affected by these reactions. It should be noted that during this reaction an ortho/para directing alkyl group is converted to a meta directing carboxylic.
Two other examples of this reaction are given below, and illustrate its usefulness in preparing substituted benzoic acids.
The mechanism of this reaction is obscure, but the fact that it specifically requires that there be a benzylic C-H bond suggests that breaking this bond is essential. Any intermediate that might be formed by breaking this bond will be stabilized by resonance with the aryl group, which provides an explanation for the specificity of attack at the benzylic position. Such reactions also occur in a biological context. Enzymes oxidize alkyl side chains on aromatic rings as part of making such compounds soluble enough to be eliminated.
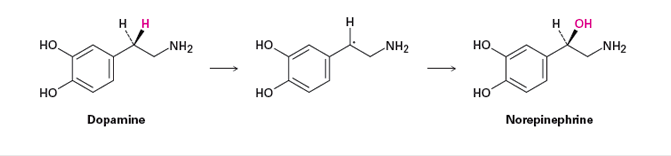
Analogous side-chain oxidations occur in various biosynthetic pathways. The neurotransmitter norepinephrine, for instance, is biosynthesized from dopamine by a benzylic hydroxylation reaction. The process is catalyzed by the copper-containing enzyme dopamine -monooxygenase and occurs by a radical mechanism. A copper–oxygen species in the enzyme first abstracts the pro-R benzylic hydrogen to give a radical, and a hydroxyl is then transferred from copper to carbon.

Bromination of the Benzylic Carbon
The bromination reaction is the N-bromosuccinamide (NBS) radical, substitution reaction previously studied. As with the oxidation reaction, one benzylic hydrogen is needed so that it can be substituted with bromine. Examples of both reactions are shown below.
The brominating reagent, N-bromosuccinimide (NBS), has proven useful for achieving allylic or benzylic substitution in CCl4 solution at temperatures below its boiling point (77 ºC). One such application is shown in the second equation. the allylic bromination with NBS is analagous to the alkane halogenation reaction (Section 10.3) since it also occurs as a radical chain reaction. The NBS serves as the source for the bromine, which is used in the initiation step to create a bromine radical that then abstracts a proton from the allylic position in the propagation step. The radical created then reacts with the NBS to to become bromiated and the cycle continues until it is terminated.
The predominance of allylic substitution over other positions comes down to bond dissociation energies. The relative bond dissociation energies are shown in the table at the top of this section. The C-H bond that we are focusing on as the point of difference for each of the energies shows that the allylic C-H bond has a strength of about 88 kcal/mol. This means that the allylic radical created is more stable than a typical alkyl radical with the same substitution by about 9 kcal/mol. Therefore, this radical is the most likely one to form and thus react.
The benzylic C-H bonds weaker than most sp3 hybridized C-H. This is because the radical formed from homolysis is resonance stabilized.

Resonance stabilization of the benzylic radical

Because of the weak C-H bonds, benzylic hydrogens can form benzylic halides under radical conditions.

NBS as a Bromine Source
NBS (N-bromosuccinimide) is the most commonly used reagent to produce low concentrations of bromine. When suspended in tetrachloride (CCl4), NBS reacts with trace amounts of HBr to produce a low enough concentration of bromine to facilitate the allylic bromination reaction.
.jpg?revision=1&size=bestfit&width=307&height=104)
Allylic Bromination Mechanism
Step 1: Initiation
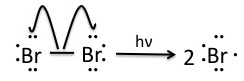
Once the pre-initiation step involving NBS produces small quantities of Br2, the bromine molecules are homolytically cleaved by light to produce bromine radicals.
Step 2 and 3: Propagation

Step 4: Termination

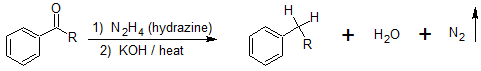
Acyl Side Chain Reductions
Since there are several limitations to the Friedel-Crafts alkylation that are not observed with the Friedel-Crafts acylation, reduction of the acyl side chain to an alkyl side chain is a useful reaction for multiple step synthesis. The Wolff-Kishner reaction reduces the carbon groups (aldehydes and ketones) to alkanes and is not limited to acyl groups bonded to benzene rings. This acyl reduction reaction is also useful because acyl groups are deactivating, meta-directors, and alkyl groups are activating, ortho-, para-directors which adds flexibility to multiple step synthesis strategies.
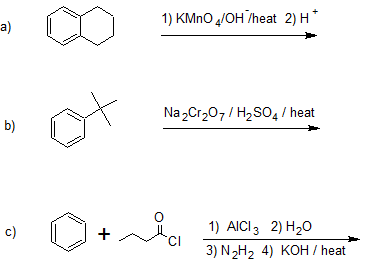
Exercise
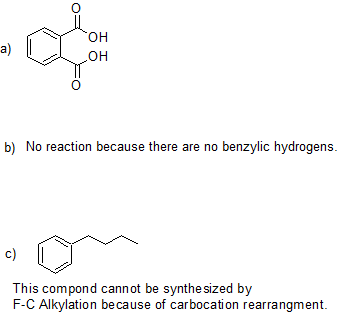
21. Draw the bond-line structures for the product(s) of the following reactions.
- Answer
-
21.




.jpg?revision=1&size=bestfit&width=307&height=104)



