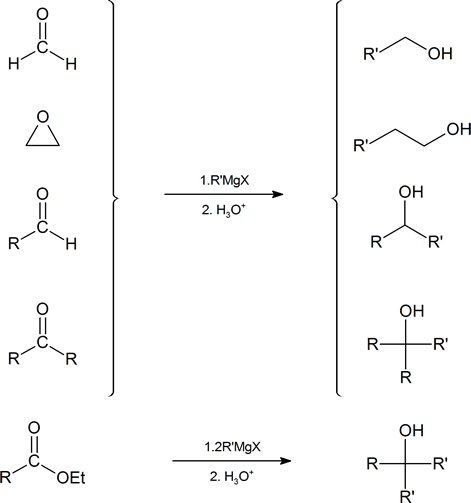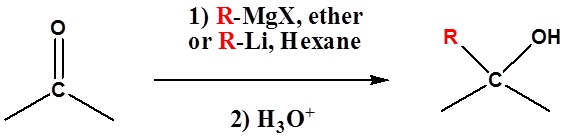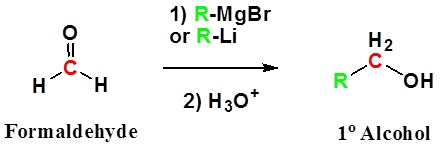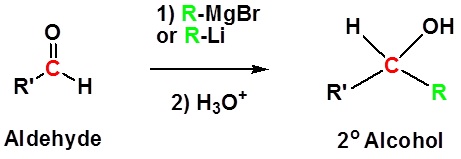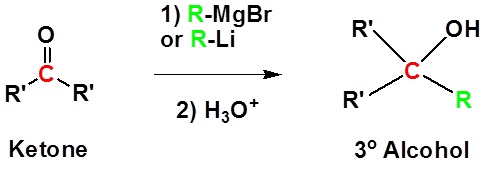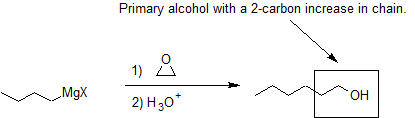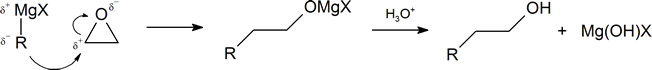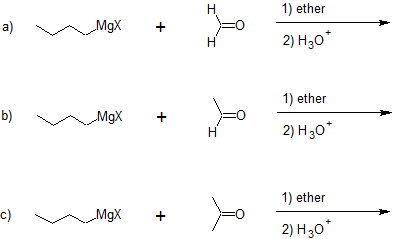Introduction
The nucleophilic carbon atoms of organometallic reagents react with the electrophilic carbon atoms of aldehydes, ketones, acyl halides, esters, and epoxides to build larger carbon chains. In the process, an alcohol is formed. The ability to build larger organic molecules is an important and useful skill for multi-step synthesis. These various reaction pathways are summarized below showing the Grignard reagent.
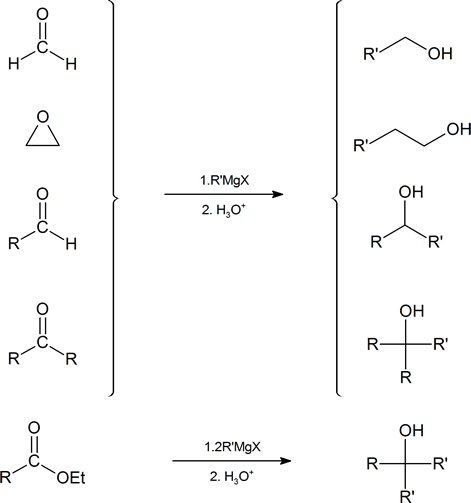
Because organometallic reagents react as their corresponding carbanion, they are excellent nucleophiles. The basic reaction involves the nucleophilic reaction of the carbanionic carbon in the organometallic reagent with the electrophilic carbon in the carbonyl to form alcohols.
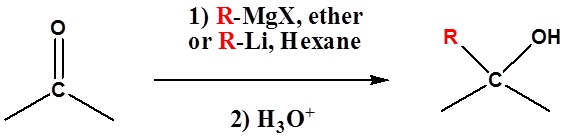
Both Grignard and Organolithium Reagents will perform these reactions.
Addition to formaldehyde gives 1o alcohols.
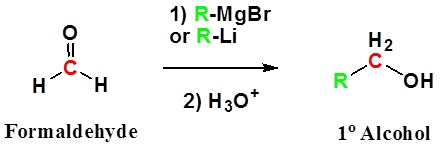
Addition to aldehydes gives 2o alcohols.
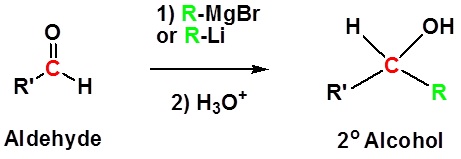
Addition to ketones gives 3o alcohols
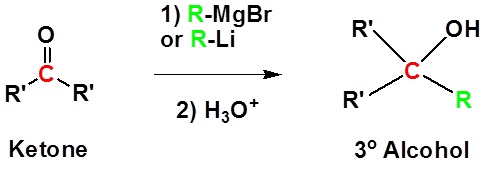
Examples
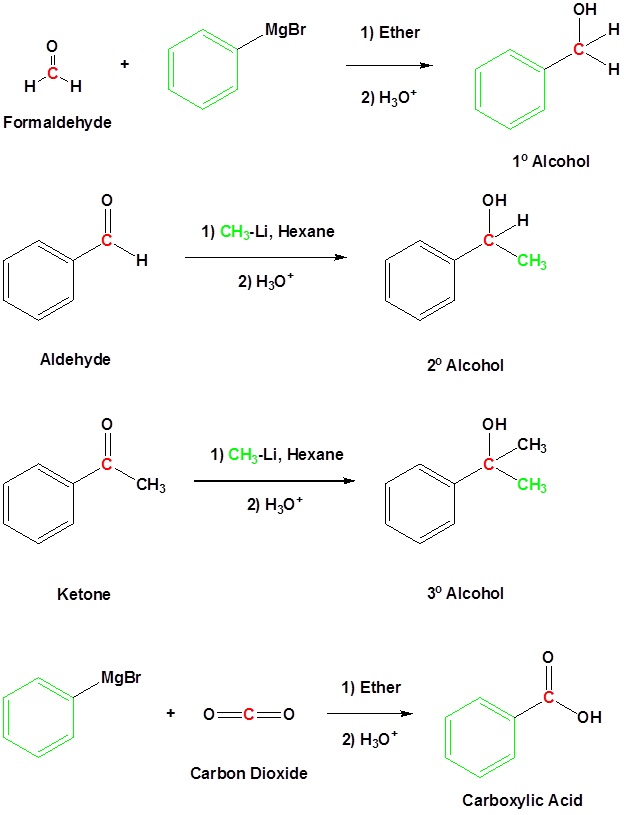
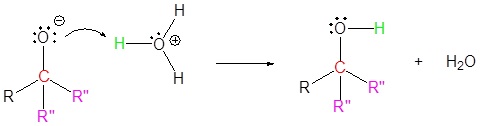
Mechanism for the Addition to Carbonyls
The mechanism for a Grignard agent is shown. The mechanism for an organolithium reagent is the same.
1) Nucleophilic reaction
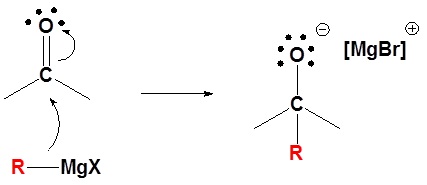
2) Protonation
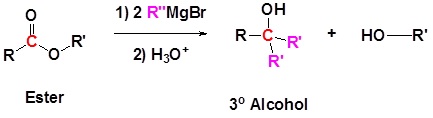
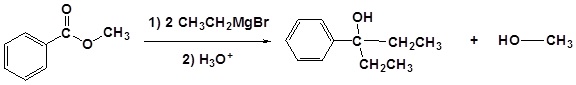
Grignard reagents convert esters to 3o alcohols
After the first Grignard reaction, the carbonyl reforms creating a ketones which can then react with the Grignard. In effect, the Grignard reagent adds twice.
Mechanism
1) Nucleophilic reaction
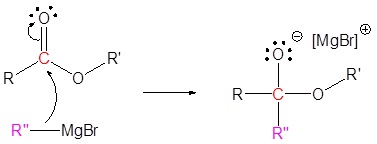
2) Carbonyl reforms with leaving group removal
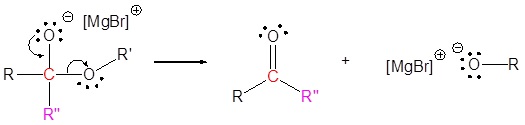
3) Nucleophilic reaction
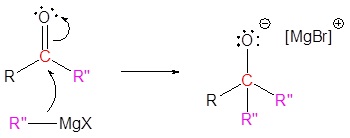
4) Protonation
Example
Grignard reagents convert Acyl Halides to 3o alcohols
Grignard reagents react with acyl halides similar to the reaction with esters. The first reaction produces a ketone which them undergoes a second reaction to form a tertiary alcohol following the analogous mechanism shown above for esters.

The reaction of benzoyl chloride with a Grignard reagent is shown below as an example.

Grignard Reactions with Epoxides
Another important route for producing an alcohol from a Grignard reagent involves the reaction of the Grignard reagent with ethylene oxide to produce a primary alcohol containing two more carbon atoms than the original Grignard reagent.
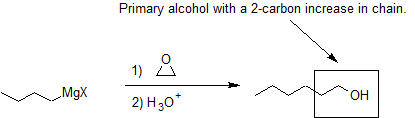
The first step of the mechanism is shown below. With the second step following the protonation step common to the other reaction pathways studied in this section.
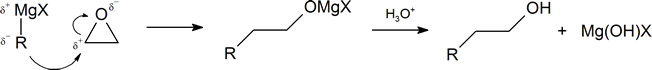
As discussed above, Grignard and organolithium reagents are powerful bases. Because of this they cannot be used as nucleophiles on compounds which contain acidic hydrogens. If they are used they will act as a base and deprotonate the acidic hydrogen rather than act as a nucleophile and attack the carbonyl. A partial list of functional groups which cannot be used are: alcohols, amides, 1o amines, 2o amines, carboxylic acids, and terminal alkynes.
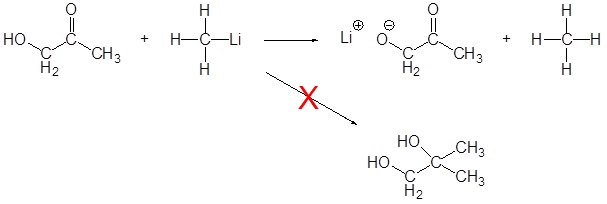
Exercises
14. Predict the products of the reactions below.
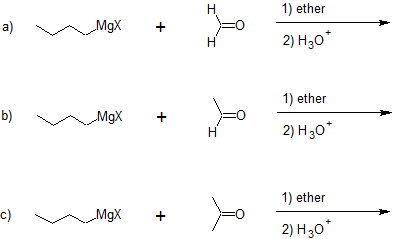
15. If allylmagnesium chloride were added to a solution of the following compound and then worked-up with acid, the product would contain a chiral center. Would the product be a racemic mixture or an enatiomerically pure product? Draw both enantiomers.
![]()
16. What combination of carbonyl compound and grignard (use MgBr) reagent would yield the following alcohols (after workup)?
(a) ![]() (b)
(b) ![]() (c)
(c) ![]() (d)
(d) ![]()
17. The following epoxide can be transformed into an alcohol using a grignard reagent, take for example allylmagnesium chloride. Draw the product of the treatment of this epoxide with this grignard after being worked up with H2O. Note the stereochemistry and also remember that benzyllic carbons are good Sn2 electrophiles.
![]()
18. How might you prepare the following molecules from esters and Grignards?
(a)![]()
(b)![]()
(c)![]()
- Answer
-
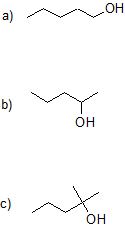
14.
15.
The result would be a racemic mixture of the following.
![]()
16.
(a) ![]()
(b) ![]()
(c) ![]()
(d) ![]()
17.
![]()
18.
(a)![]()
(b) ![]()
(c)![]()
Contributors and Attributions