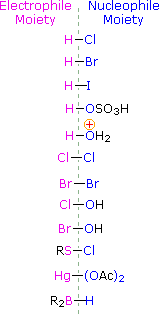Introduction
The proton is not the only electrophilic species that initiates addition reactions to the double bond of alkenes. Lewis acids like the halogens, boron hydrides and certain transition metal ions are able to accept the alkene pi-electrons. The resulting positively charged intermediates attract nucleophiles to give addition products.The electrophilic character of the halogens is well known. Fluorine adds uncontrollably with alkenes, and the addition of iodine is unfavorable, so these are not useful preparative methods. Chlorine (Cl2) and bromine (Br2) react selectively with the double bond of alkenes, so we will focus on these reactions.
The addition of chlorine and bromine to alkenes, as shown below, produces vicinal dihalo-compounds. In this reaction, we can assume that the solvent was something that is not nucleophilic, such as tetrahydrofuran (THF).
R2C=CR2 + X2 ——> R2CX-CR2X
If this same reaction is performed in a nucleophilic solvent like water or an alcohol, then the solvent becomes the nucleophile in the second step and reacts with the bromonium (or chloronium) ion to form a halohydrin as shown below.
R2C=CR2 + X2 + H2O ——> R2COH-CR2X
There are also other halogen-containing reagents that add to double bonds, such as hypohalous acids, HOX, and sulfenyl chlorides, RSCl. These reagents are unsymmetrical, so their addition to unsymmetrical double bonds may in principle take place in two ways. In practice, these addition reactions are regioselective, with one of the two possible constitutionally isomeric products being favored. The electrophilic moiety in both of these reagents is the halogen.
(CH3)2C=CH2 + HOBr ——> (CH3)2COH-CH2Br
(CH3)2C=CH2 + C6H5SCl ——> (CH3)2CCl-CH2SC6H5
Mechanisms explain the Regioselectivity
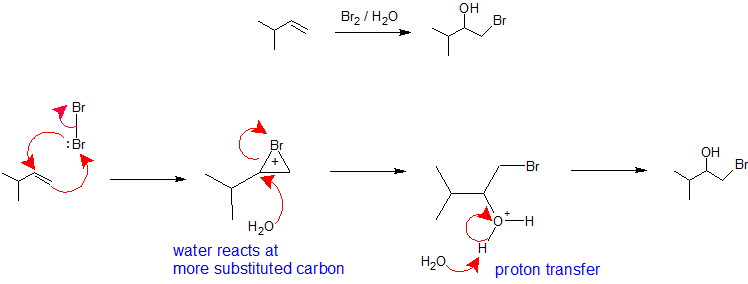
X2/H2O or X2/ROH: The regioselectivity of halohydrin formation from an alkene reaction with a halogen in a nucleophilic solvent is analogous to the oxymercuration-demercuration pathway. The halogen molecule takes the role of electrophile accepting nucleophilic pi electrons from the alkene while simultaneously forming a bond with the other vinyl carbon to create a bromonium (or chloroium) ion. The bromonium (or chloronium) ion formation stabilizes the positive charge and prevents carbocation rearrangement. The solvent takes the role of the nucleophile because it is present is a much greater percentage than the leaving group and reacts with the most substituted carbon of the cyclic bromonium (or chloronium) ion to create regiochemistry. The stereochemistry of this reaction is anti-addition because the solvent approaches the bromonium ion with back side orientation to produce the addition product. However, since the interaction of the halogen with the alkene can occur from above or below, there is no stereochemical control in this reaction and a mixture of enantiomers will be produced when applicable. The final step of this mechanism is a proton transfer to a solvent water molecule to neutralize the addition product.
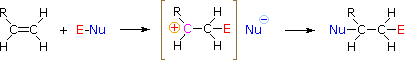
HOX or RSCl: The regioselectivity of the hypohalous acids and sulfenyl chloride reactions may be explained by the same mechanism we used to rationalize the Markovnikov rule. Bonding of an electrophilic species to the double bond of an alkene forms preferentially to produce the more stable (more highly substituted) carbocation. This intermediate should then combine rapidly with a nucleophilic species to produce the addition product.
To apply this mechanism we need to determine the electrophilic moiety in each of the reagents. By using electronegativity differences we can dissect common addition reagents into electrophilic and nucleophilic moieties, as shown on the right. In the case of hypochlorous and hypobromous acids (HOX), these weak Brønsted acids (pKa's ca. 8) do not react as proton donors; and since oxygen is more electronegative than chlorine or bromine, the electrophile will be a halide cation. The nucleophilic species that bonds to the intermediate carbocation is then hydroxide ion, or more likely water (the usual solvent for these reagents), and the products are called halohydrins. Sulfenyl chlorides add in the opposite manner because the electrophile is a sulfur cation, RS(+), whereas the nucleophilic moiety is chloride anion (chlorine is more electronegative than sulfur).
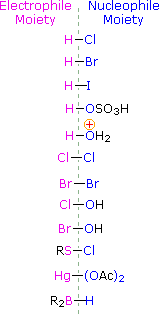
Below are some examples illustrating the addition of various electrophilic halogen reagents to alkene groups. Notice the specific regiochemistry of the products, as explained above.

Exercise
1. Predict the product of the following reaction:
![]()
2. When butene is treated with NBS in the presence of water, the product shows that the bromine is on the least substituted carbon, is this Markovnikov or anti-Markovnikov?
- Answer
-
1.
![]()
2. Since the bromine is the first addition to the alkene, this addition would be an anti-Markovnikov addition.