Predicting SN1 vs. SN2 mechanisms
When considering whether a nucleophilic substitution is likely to occur via an SN1 or SN2 mechanism, we really need to consider three factors:
1) The electrophile: when the leaving group is attached to a methyl group or a primary carbon, an SN2 mechanism is favored (here the electrophile is unhindered by surrounded groups, and any carbocation intermediate would be high-energy and thus unlikely). When the leaving group is attached to a tertiary, allylic, or benzylic carbon, a carbocation intermediate will be relatively stable and thus an SN1 mechanism is favored. These patterns of reactivity of summarized below.
| Alkyl Halide Structure |
Possible Substitution Reactions |
| methyl and primary |
SN2 only |
| secondary |
SN2 and SN1 |
| tertiary |
SN1 only |
| primary and secondary benzylic and allylic |
SN2 and SN1 |
| tertiary benzylic and allylic |
SN1 only |
| vinyl and aryl |
NO reaction |
2) The nucleophile: powerful nucleophiles, especially those with negative charges, favor the SN2 mechanism. Weaker nucleophiles such as water or alcohols favor the SN1 mechanism.
3) The solvent: Polar aprotic solvents favor the SN2 mechanism by enhancing the reactivity of the nucleophile. Polar protic solvents favor the SN1 mechanism by stabilizing the transition state and carbocation intermediate. SN1 reactions are called solvolysis reactions when the solvent is the nucleophile.
These patterns of reactivity are summarized in the table below.
Comparison between SN2 and SN1 Reactions
| Reaction Parameter |
SN2 |
SN1 |
| alkyl halide structure |
methyl > primary > secondary >>>> tertiary |
tertiary > secodary >>>> primary > methyl |
| nucleophile |
high concentration of a strong nucleophile |
poor nucleophile (often the solvent) |
| mechanism |
1-step |
2-stp |
| rate limiting step |
bimolecular transition state |
carbocation formation |
| rate law |
rate = k[R-X][Nu] |
rate = k[R-X] |
| stereochemisty |
inversion of configuration |
mixed configuration |
| solvent |
polar aprotic |
polar protic |
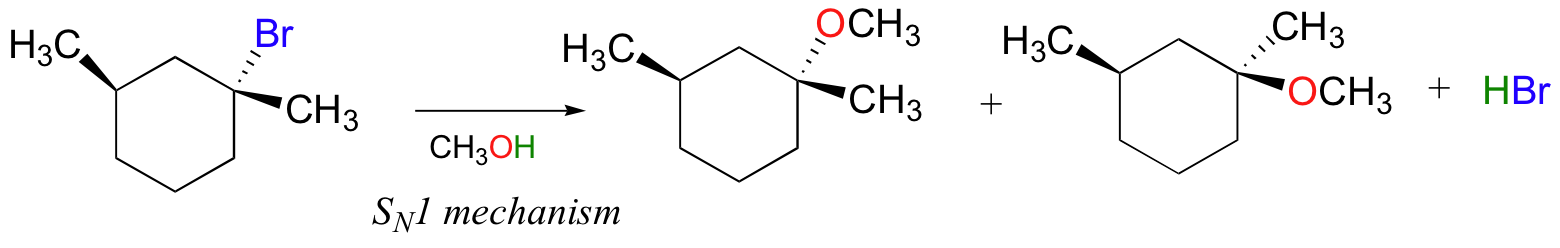
For example, the reaction below has a tertiary alkyl bromide as the electrophile, a weak nucleophile, and a polar protic solvent (we’ll assume that methanol is the solvent). Thus we’d confidently predict an SN1 reaction mechanism. Because substitution occurs at a chiral carbon, we can also predict that the reaction will proceed with racemization.
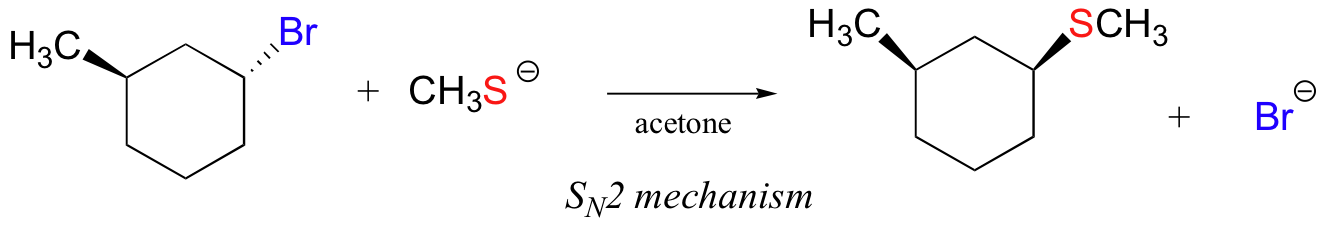
In the reaction below, on the other hand, the electrophile is a secondary alkyl bromide – with these, both SN1 and SN2 mechanisms are possible, depending on the nucleophile and the solvent. In this example, the nucleophile (a thiolate anion) is strong, and a polar protic solvent is used – so the SN2 mechanism is heavily favored. The reaction is expected to proceed with inversion of configuration.
Exercise
1. Determine whether each substitution reaction shown below is likely to proceed by an SN1 or SN2 mechanism and explain your reasoning.
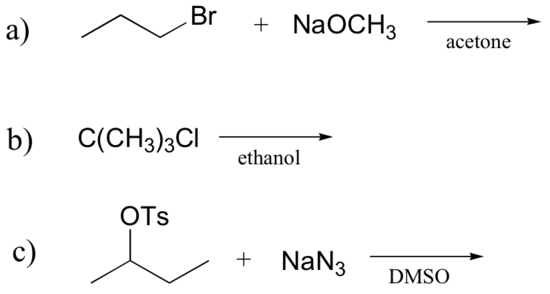
- Answer
-
a) SN2 b/c primary alkyl halide with a strong nucleophile in a polar aprotic solvent.
b) SN1 b/c tertiary alkyl halide with a weak nucleophile that is also the solvent (solvolysis).
c) SN2 b/c secondary alkyl halides favor this mechanism when reacted with a strong nucleophile (and weak base) in a polar aprotic solvent.




