6.9: Characteristics of the Sₙ1 Reaction
- Last updated
- Save as PDF
- Page ID
- 182908
Learning Objective
- determine the rate law & predict the mechanism based on its rate equation or reaction data for SN1 reactions
- predict the products and specify the reagents for SN1 reactions with stereochemistry
- propose mechanisms for SN1 reactions
- draw and interpret Reaction Energy Diagrams for SN1 reactions
In order of decreasing importance, the factors impacting SN1 reaction pathways are
- structure of the alkyl halide
- stability of the leaving group
- type of solvent.
The unimolecular transition state of the SN1 pathway means that structure of the alkyl halide and stability of the leaving group are the primary considerations. Alkyl halides that can ionize to form stable carbocations are more reactive via the SN1 mechanism. Because carbocation stability is the primary energetic consideration, stabilization of the carbocation via solvation is also an important consideration.
Alkyl Halide Structure and Carbocation Stability
The first order kinetics of SN1 reactions suggest a two-step mechanism in which the rate-determining step consists of carbocation formation from the ionization of the alkyl halide as shown in the diagram below. In this mechanism, the carbocation is a high-energy intermediate the bonds immediately to nearby nucleophiles. The only reactant that is undergoing change in the first (rate-determining) step is the alkyl halide, so we expect such reactions would be unimolecular and follow a first-order rate equation. Hence the name SN1 is applied to this mechanism.
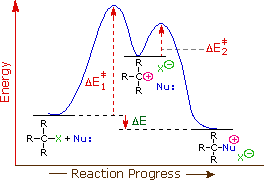
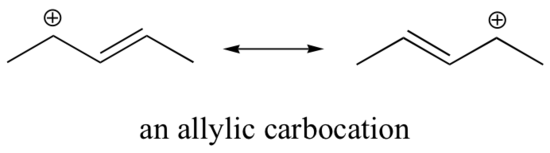
The Hammond postulate suggests that the activation energy of the rate-determining first step will be inversely proportional to the stability of the carbocation intermediate: the more stable the carbocation, the lower the activation energy, the faster the reactivity. Therefore, carbocation stability is a primary consideration in SN1 reactions. Carbocations can be stabilized by delocalizing the charge via resonance and through inductive electron donation of alkyl groups. Carbocations can also stabilize by rearrangement via 1,2-hydride or 1,2-methyl shifts. Carbocation rearrangements are explained in a subsequent section of this chapter.
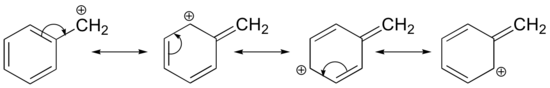
Benzyl Carbocation
The relative stability of carbocations is summarized below.

|
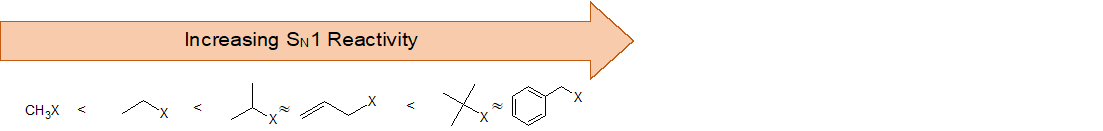
Consequently, we expect that 3º-alkyl halides will be more reactive than their 2º and 1º-counterparts in reactions that follow an SN1 mechanism. This is opposite to the reactivity order observed for the SN2 mechanism. Allylic and benzylic halides are exceptionally reactive by either mechanism. This trend is summarized in the diagram below.
Effects of Leaving Group
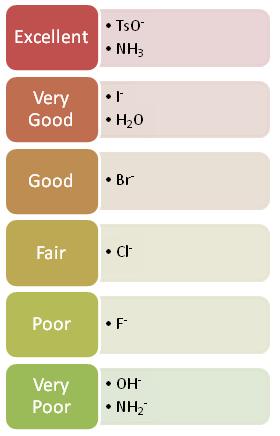
An SN1 reaction speeds up with a good leaving group. This is because the leaving group is involved in the rate-determining step. A good leaving group wants to leave so it breaks the C-Leaving Group bond faster. Once the bond breaks, the carbocation is formed and the faster the carbocation is formed, the faster the nucleophile can come in and the faster the reaction will be completed.
A good leaving group is a weak base because weak bases can hold the charge. They're happy to leave with both electrons and in order for the leaving group to leave, it needs to be able to accept electrons. Strong bases, on the other hand, donate electrons which is why they can't be good leaving groups. As you go from left to right on the periodic table, electron donating ability decreases and thus ability to be a good leaving group increases. Halides are an example of a good leaving group whos leaving-group ability increases as you go down the column.
.jpg?revision=1&size=bestfit&width=300&height=74)
The two reactions below is the same reaction done with two different leaving groups. One is significantly faster than the other. This is because the better leaving group leaves faster and thus the reaction can proceed faster.
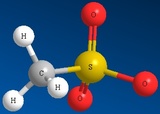
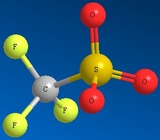
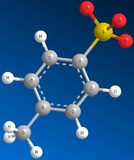
Methyl Sulfate Ion Mesylate Ion Triflate Ion Tosylate Ion
CH3SO42- CH3SO32- CF3SO32- CH3C6H4SO32-
Solvent Effects on the SN1 Reaction
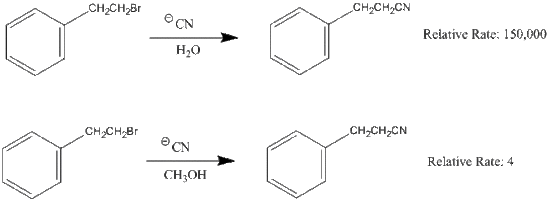
To facilitate the charge separation of the ionization reaction in the first step, a good ionizing solvent is needed. Two solvent characteristics will be particularly important - the polarity and the solvating power. The dielectric constant, ε, measures polarity of solvent molecules and their ability to orient themselves between ions to attenuate (reduce) the electrostatic force one ion exerts on the other. The higher the dielectric constant the more polar the substance and in the case of SN1 reactions, the faster the rate. A dielectric constant below 15 is usually considered non-polar. Solvents having high dielectric constants, such as water (ε=81), formic acid (ε=58), dimethyl sulfoxide (ε=45) & acetonitrile (ε=39) are generally considered better ionizing solvents than are some common organic solvents such as ethanol (ε=25), acetone (ε=21), methylene chloride (ε=9) & ether (ε=4). Below is the same reaction conducted in two different solvents. The relative reaction rate in water (ε=81) is 150,000 times faster than in methanol (ε=33).
Solvation refers to the solvent's ability to stabilize ions by encasing them in a sheath of weakly bonded solvent molecules. Anions are solvated by partial positive charges of hydrogen-bonding solvents. Cations are often best solvated by the nucleophilic sites on a solvent molecule (e.g. oxygen & nitrogen atoms). The interaction of the carbocations with these nucleophilic solvents may be strong enough to form covalent bonds to carbon, thus converting the intermediate to a substitution product and creating the reaction name "solvolysis". When solvolysis occurs with water, the actions are called "hydrolysis reactions" as shown in the reaction below.
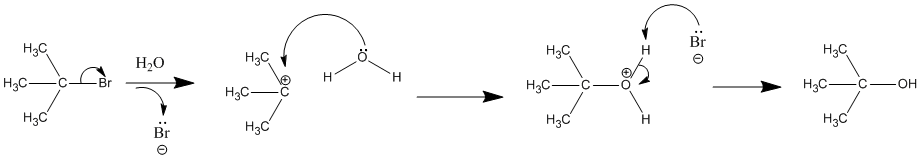
Polar Protic and Polar Aprotic Solvents
Protic solvents contain polarized hydrogen. Whereas, aprotic solvents do NOT contain polarized hydrogen. For SN2 reactions, solvation of the nucleophile by polar protic solvents slows the reaction rate. However, in SN1 reaction the nucleophile is not a part of the rate-determining step so this concern is not relevant. In fact, polar protic solvents actually speed up the rate of SN1 reactions because the polar solvent helps stabilize the transition state and carbocation intermediate. Since the carbocation is unstable, anything that can stabilize this even a little will speed up the reaction. Polar aprotic solvents have a dipole moment, but their hydrogen is not highly polarized. Polar aprotic solvents are not used in SN1 reactions because some of them can react with the carbocation intermediate and give an unwanted side-product. Rather, polar protic solvents are preferred for unimolecular substitution reactions.
Effects of Nucleophile
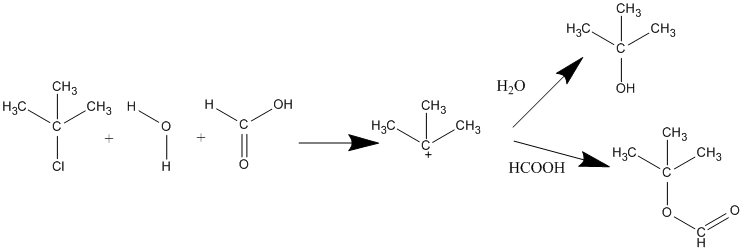
The strength of the nucleophile does not affect the reaction rate of SN1 because the nucleophile is not involved in the rate-determining step. Since nucleophiles only participate in the fast second step, their relative molar concentrations rather than their nucleophilicities should be the primary product-determining factor. If a nucleophilic solvent such as water is used, its high concentration will assure that alcohols are the major product. However, if you have more than one nucleophile competing to bond to the carbocation, the strengths and concentrations of those nucleophiles affects the distribution of products. For example, if t-butylchloride reacts with a mixture of water and formic acid where the water and formic acid are competing nucleophiles, two different products are formed: (CH3)3COH and (CH3)3COCOH. The relative yields of these products depends on the concentrations and relative reactivities of the nucleophiles. With a higher electron density, water is considered the stronger nucleophile and the tertiary alcohol will be the major product if there are equal concentrations of competing nucleophiles.
Exercises
1. Rank the following by increasing reactivity in an SN1 reaction.
2. 3-bromo-1-pentene and 1-bromo-2-pentene undergo SN1 reaction at almost the same rate, but one is a secondary halide while the other is a primary halide. Explain why this is.
3. Label the following reactions as most likely occuring by an SN1 or SN2 mechanism. Suggest why.
- Answers
-
1. Consider the stability of the intermediate, the carbocation.
A < D < B < C (most reactive)
2. They have the same intermediates when you look at the resonance forms.
3. A – SN1 *poor leaving group, protic solvent, secondary cation intermediate
B – SN2 *good leaving group, polar solvent, primary position.