Introduction
Because axial bonds are parallel to each other, substituents larger than hydrogen generally suffer greater steric crowding when they are oriented axial rather than equatorial. Consequently, substituted cyclohexanes will preferentially adopt conformations in which the larger substituents assume equatorial orientation.
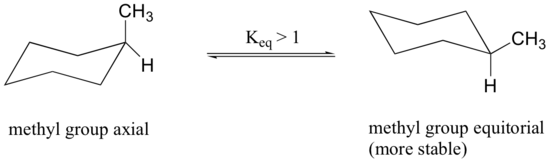
When the methyl group in the structure above occupies an axial position it suffers steric crowding by the two axial hydrogens located on the same side of the ring. The conformation in which the methyl group is equatorial is more stable, and thus the equilibrium lies in this direction.
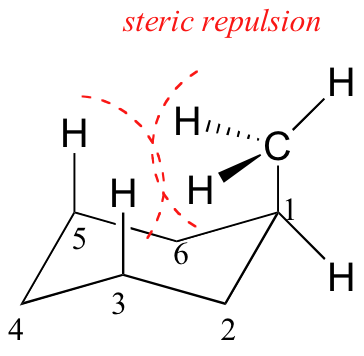
In examining possible structures for monosubstituted cyclohexanes, it is useful to follow two principles:
- Chair conformations are generally more stable than other possibilities.
- Substituents on chair conformers prefer to occupy equatorial positions due to the increased steric hindrance of axial locations.
Experimental Measurements of Steric Hindrance
The relative steric hindrance experienced by different substituent groups oriented in an axial versus equatorial location on cyclohexane may be determined by the conformational equilibrium of the compound. The corresponding equilibrium constant is related to the energy difference between the conformers and collecting such data allows us to evaluate the relative tendency of substituents to exist in an equatorial or axial location.
Looking at the energy values the table, it is clear that the apparent "size" of a substituent (in terms of its preference for equatorial over axial orientation) is influenced by its width and bond length to cyclohexane, as evidenced by the fact that an axial vinyl group is less hindered than ethyl, and iodine slightly less than chlorine.
A Selection of AG° Values for the Change from Axial to Equatorial Orientation of Substituents for Monosubstituted Cyclohexanes
| Substituent |
\(-\Delta{G}^o\) kcal/mol |
Substituent |
\(-\Delta{G}^o\) kcal/mol |
| \(\ce{CH_3\bond{-}}\) |
1.7 |
\(\ce{O_2N\bond{-}}\) |
1.1 |
| \(\ce{CH_2H_5\bond{-}}\) |
1.8 |
\(\ce{N#C\bond{-}}\) |
0.2 |
| \(\ce{(CH_3)_2CH\bond{-}}\) |
2.2 |
\(\ce{CH_3O\bond{-}}\) |
0.5 |
| \(\ce{(CH_3)_3C\bond{-}}\) |
\(\geq 5.0\) |
(CH3)3C- |
0.7 |
| \(\ce{F\bond{-}}\) |
0.3 |
F- |
1.3 |
| \(\ce{Cl\bond{-}}\) |
0.5 |
\(\ce{C_6H_5\bond{-}}\) |
3.0 |
| \(\ce{Br\bond{-}}\) |
0.5 |
|
|
| \(\ce{I\bond{-}}\) |
0.5 |
|
|
Exercise
1. In the molecule, cyclohexyl ethyne there is little steric strain, why?
- Answer
-
1.
The ethyne group is linear and therefore does not affect the hydrogens in the 1,3 positions to say to the extent as a bulkier or a bent group (e.g. ethene group) would. This leads to less of a strain on the molecule.
![]()



