Saturated vs. Unsaturated Molecules
Hydrocarbons are organic compounds that contain only carbon and hydrogen. The broadest distinction between hydrocarbons is whether they are saturated and unsaturated. Saturated hydrocarbons only contain carbon-carbon single bonds with the maximum number of hydrogens relative to the number of carbon atoms. It can be said that the carbon atoms are "saturated" with hydrogen atoms in the same way a saturated solution has dissolved the maximum amount of solute. Hydrocarbons that contain pi bonds as carbon-carbon double or triple bonds are classified as unsaturated hydrocarbons. Unsaturation indicates that some of the carbon-hydrogen bonds were lost to from pi bonds between carbon atoms. There are less than the maximum number os hydrogens relative to the number of carbon atoms.
- Saturated hydrocarbons (alkanes) are the simplest of the hydrocarbon species. They are composed entirely of single bonds and are saturated with hydrogen. Saturated hydrocarbons are the basis of petroleum fuels and are found as either linear or branched species.The simplest alkanes have their C atoms bonded in a straight chain; these are called normal alkanes. They are named according to the number of C atoms in the chain. The smallest alkane is methane:
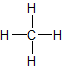
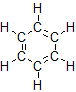
2. Unsaturated hydrocarbons have one or more double or triple bonds between carbon atoms. Those with double bond are called alkenes and those with one double bond have the formula \(C_nH_{2n}\) (assuming non-cyclic structures). Those containing triple bonds are called alkynes, with general formula CnH2n-2.
The smallest alkene—ethene—has two C atoms and is also known by its common name ethylene:
The smallest alkyne is ethyne, which is also known as acetylene:
Hydrocarbon Functional Groups
The four distinct hydrocarbon functional groups are: alkanes, alkenes, alkynes and arenes. Aromatic compounds derive their names from the fact that many of these compounds in the early days of discovery were grouped because they were oils with fragrant odors.
Alkanes are organic compounds that consist entirely of single-bonded carbon and hydrogen atoms and lack any other functional groups. Alkanes have the general formula CnH2n+2. Alkanes can be subdivided into the following three groups: the linear straight-chain alkanes, branched alkanes, and cycloalkanes. Alkanes are also saturated hydrocarbons. Alkanes are the simplest and least reactive hydrocarbon species containing only carbons and hydrogens. The distinguishing feature of an alkane, making it distinct from other compounds that also exclusively contain carbon and hydrogen, is its lack of unsaturation. That is to say, it contains no double or triple bonds, which are highly reactive in organic chemistry. Though not totally devoid of reactivity, their lack of reactivity under most laboratory conditions makes them a relatively uninteresting, though very important component of organic chemistry. As you will learn about later, the energy confined within the carbon-carbon bond and the carbon-hydrogen bond is quite high and their rapid oxidation produces a large amount of heat, typically in the form of fire.
The general formula for saturated hydrocarbons is CnH2n+2(assuming non-cyclic structures) as shown in hexane (C6H14) below.
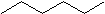
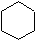
Cycloalkanes are hydrocarbons containing one or more carbon rings to which hydrogen atoms are attached. The general formula for a cyclic hydrocarbon containing one ring is CnH2n as shown in cyclohexane (C6H12) below.
Akenes contain at least one carbon-carbon double bond and alkynes contain at least one carbon-carbon triple bond. Alkenes have the general formula CnH2n. Alkynes have the general formula CnH2n-2. The ratio of carbon to hydrogen increased because hydrogen atoms are replaced with pi bonds as shown in trans-2-butene (C4H8) and 2-butyne (C4H6) below. Since both double and triple bonds include pi bonds, Alkenes and alkynes share similar chemical reactivity.

Aromatic hydrocarbons, also known as arenes, are hydrocarbons that have at least one aromatic ring. Aromatic compounds contain the benzene unit. Benzene itself is composed of six C atoms in a ring, with alternating single and double C–C bonds:

Calculating Degrees of Unsaturation (DU)
There are many ways one can go about determining the structure of an unknown organic molecule. Although, nuclear magnetic resonance (NMR) and infrared radiation (IR) are the primary ways of determining molecular structures, calculating the degrees of unsaturation (DU) is useful information. Knowing the degrees of unsaturation tells us the combined number of pi bonds and rings within a compound which makes it easier to figure out the molecular structure.
Degree of Unsaturation (DU) can be calculated with the equation below and the molecular formula
DU= (2C+2+N-X-H)/2
where: C is the number of carbons; N is the number of nitrogens; X is the number of halogens (F, Cl, Br, I); and H is the number of hydrogens from the molecular formula.
As stated before, a saturated molecule contains only single bonds and no rings. Another way of interpreting this is that a saturated molecule has the maximum number of hydrogen atoms possible to be an acyclic alkane. Thus, the number of hydrogens can be represented by 2C+2, which is the general molecular representation of an alkane. As an example, for the molecular formula C3H4 the number of actual hydrogens needed for the compound to be saturated is 8 [2C+2=(2x3)+2=8]. The compound needs 4 more hydrogens in order to be fully saturated (expected number of hydrogens-observed number of hydrogens=8-4=4). Degrees of unsaturation is equal to 2, or half the number of hydrogens the molecule needs to be classified as saturated. Hence, the DoB formula divides by 2. The formula subtracts the number of X's because a halogen (X) replaces a hydrogen in a compound. For instance, in chloroethane, C2H5Cl, there is one less hydrogen compared to ethane, C2H6.
For a compound to be saturated, there is one more hydrogen in a molecule when nitrogen is present. Therefore, we add the number of nitrogens (N). This can be seen with C3H9N compared to C3H8. Oxygen and sulfur are not included in the formula because saturation is unaffected by these elements. As seen in alcohols, the same number of hydrogens in ethanol, C2H5OH, matches the number of hydrogens in ethane, C2H6.
The following chart illustrates the possible combinations of the number of double bond(s), triple bond(s), and/or ring(s) for a given degree of unsaturation. Each row corresponds to a different combination.
- One degree of unsaturation is equivalent to 1 ring or 1 double bond (1 \( \pi \) bond).
- Two degrees of unsaturation is equivalent to 2 double bonds, 1 ring and 1 double bond, 2 rings, or 1 triple bond (2 \( \pi \) bonds).
|
DU
|
Possible combinations of rings/ bonds
|
|
|
# of rings
|
# of double bonds
|
# of triple bonds
|
|
1
|
1
|
0
|
0
|
|
|
0
|
1
|
0
|
|
2
|
2
|
0
|
0
|
|
|
0
|
2
|
0
|
|
|
0
|
0
|
1
|
|
|
1
|
1
|
0
|
| 3 |
3 |
0 |
0 |
| |
2 |
1 |
0 |
| |
1 |
2 |
0 |
| |
0 |
1 |
1 |
| |
0 |
3 |
0 |
| |
1 |
0 |
1 |
Remember, the degrees of unsaturation only gives the sum of double bonds, triple bonds and/or rings. For instance, a degree of unsaturation of 3 can contain 3 rings, 2 rings+1 double bond, 1 ring+2 double bonds, 1 ring+1 triple bond, 1 double bond+1 triple bond, or 3 double bonds.
Example
What is the Degree of Unsaturation for benzene?
Solution - "Thinking it through"
The molecular formula for benzene is C6H6. Thus,
DU= 4, where C=6, N=0,X=0, and H=6. 1 DU can equal 1 ring or 1 double bond. This corresponds to benzene containing 1 ring and 3 double bonds.
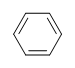
Even though there are other possible structures with a DU = 4, like the ones shown below. We will learn the benzene rings have unusual stability and occur frequently in the world of organic chemistry. When the DU for a compound is > 4, we can assume the presence of at least one benzene ring.

Exercise
- Are the following molecules saturated or unsaturated:
-
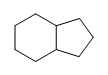 (b.)
(b.) (c.)
(c.) 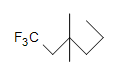 (d.) C10H6N4
(d.) C10H6N4
- Using the molecules from 1., give the degrees of unsaturation for each.
- Calculate the degrees of unsaturation for the following molecular formulas:
- (a.) C9H20 (b.) C7H8 (c.) C5H7Cl (d.) C9H9NO4
- Using the molecular formulas from 3, are the molecules unsaturated or saturated.
- Using the molecular formulas from 3, if the molecules are unsaturated, how many rings/double bonds/triple bonds are predicted?
- Answer
-
1.
(a.) unsaturated (Even though rings only contain single bonds, rings are considered unsaturated.)
(b.) unsaturated
(c.) saturated
(d.) unsaturated
2. If the molecular structure is given, the easiest way to solve is to count the number of double bonds, triple bonds and/or rings. However, you can also determine the molecular formula and solve for the degrees of unsaturation by using the formula.
(a.) 2
(b.) 2 (one double bond and the double bond from the carbonyl)
(c.) 0
(d.) 10
3. Use the formula to solve
(a.) 0
(b.) 4
(c.) 2
(d.) 6
4.
(a.) saturated
(b.) unsaturated
(c.) unsaturated
(d.) unsaturated
5.
(a.) 0 (Remember-a saturated molecule only contains single bonds)
(b.) The molecule can contain any of these combinations (i) 4 double bonds (ii) 4 rings (iii) 2 double bonds+2 rings (iv) 1 double bond+3 rings (v) 3 double bonds+1 ring (vi) 1 triple bond+2 rings (vii) 2 triple bonds (viii) 1 triple bond+1 double bond+1 ring (ix) 1 triple bond+2 double bonds
(c.) (i) 1 triple bond (ii) 1 ring+1 double bond (iii) 2 rings (iv) 2 double bonds
(d.) (i) 3 triple bonds (ii) 2 triple bonds+2 double bonds (iii) 2 triple bonds+1 double bond+1 ring (iv)... (As you can see, the degrees of unsaturation only gives the sum of double bonds, triple bonds and/or ring. Thus, the formula may give numerous possible structures for a given molecular formula.)



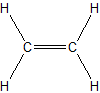
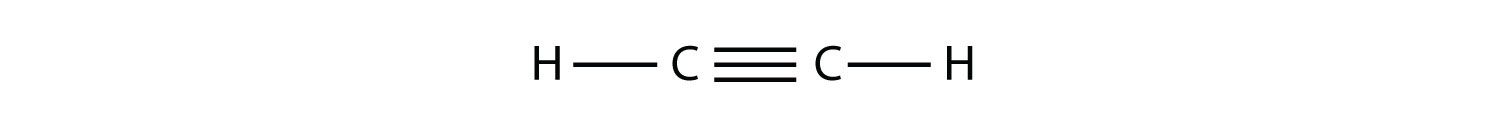

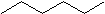






 (b.)
(b.) (c.)
(c.)  (d.) C10H6N4
(d.) C10H6N4