Claisen condensation - an overview
Recall the general mechanism for a nucleophilic acyl substitution mechanism, which we studied in chapter 10:
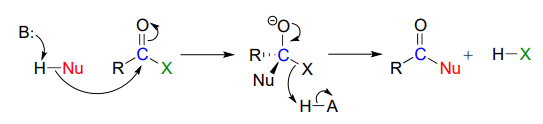
The major points to recall are that a nucleophile attacks a carboxylic acid derivative, leading to a tetrahedral intermediate, which then collapses to expel the leaving group (X). The whole process results in the formation of a different carboxylic acid derivative.
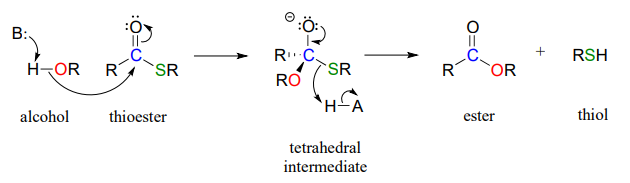
A typical nucleophilic acyl substitution reaction might have an alcohol nucleophile attacking a thioester, driving off a thiol and producing an ester.
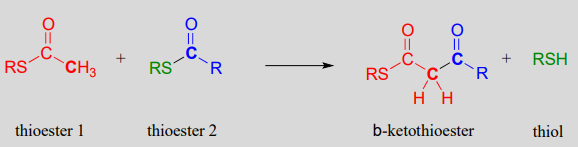
If, however, the attacking nucleophile in an acyl substitution reaction is the a-carbon of an enolate, a new carbon-carbon bond is formed. This type of reaction is called a Claisen condensation, after the German chemist Ludwig Claisen (1851-1930).
A Claisen condensation reaction
Mechanism:
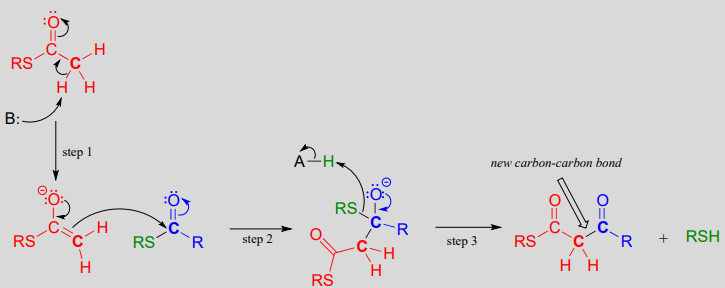
In step 1, the \(\alpha\)carbon of one thioester substrate is deprotonated to form an enolate, which then goes on to attack the second thioester substrate (step 2). Then the resulting tetrahedral intermediate collapses (step 3), expelling the thiol leaving group and leaving us with a \(\beta\)-keto thioester product (a thioester with a ketone group two carbons away).
To reiterate: A Claisen condensation reaction is simply a nucleophilic acyl substitution (Chapter 11) reaction with an enolate carbon nucleophile.
Biochemical Claisen condensation examples
A Claisen condensation between two acetyl \(CoA\) molecules (EC 2.3.1.9) serves as the first step in the biosynthesis of cholesterol and other isoprenoid compounds in humans (see section 1.3 for a reminder of what isoprenoids are). First, a transthioesterase reaction transfers the acetyl group of the first acetyl \(CoA\) to a cysteine side chain in the enzyme's active site (steps a, b). (This preliminary event is typical of many enzyme-catalyzed Claisen condensation reactions, and serves to link the electrophilic substrate covalently to the active site of the enzyme).
In the 'main' part of the Claisen condensation mechanism, the \(\alpha\)-carbon of a second acetyl \(CoA\) is deprotonated (step 1), forming a nucleophilic enolate.
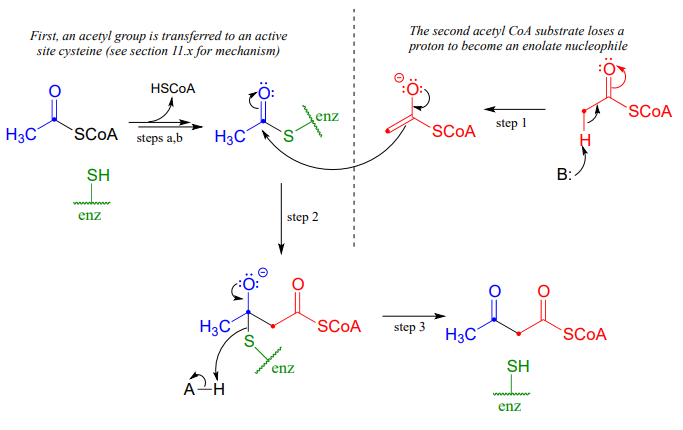
The enolate carbon attacks the electrophilic thioester carbon, forming a tetrahedral intermediate (step 2) which collapses to expel the cysteine thiol (step 3).
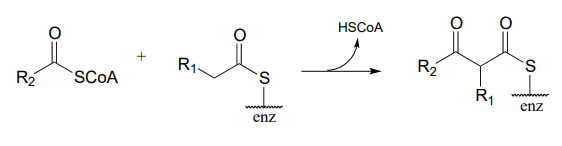
Draw curved arrows for the carbon-carbon bond-forming step in mechanism for this condensation reaction between two fatty acyl-thioester substrates. R1 and R2 can be hydrocarbon chains of various lengths. (J. Biol. Chem. 2011, 286, 10930.)
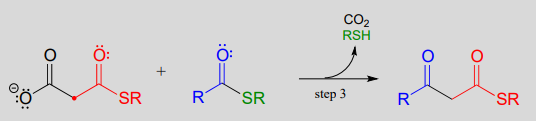
In an alternative mechanism, Claisen condensations in biology are often initiated by decarboxylation at the \(\alpha\)-carbon of a thioester, rather than by deprotonation:
Decarboxylation/Claisen condensation:
Mechanism:
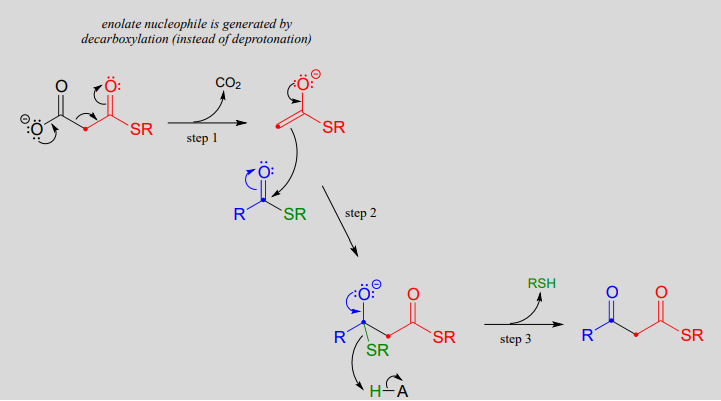
The thing to notice here is that the nucleophilic enolate (in red) is formed in the first step by decarboxylation, rather than by deprotonation of an \(\alpha\)-carbon. Other than that, the reaction looks just like the Claisen condensation reactions we saw earlier.
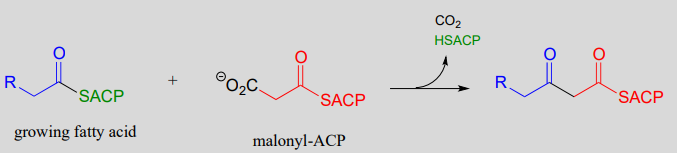
Now, we can finally understand the fatty acid chain-elongation step that we heard about in the chapter introduction in the context of the Lorenzo's oil story, which is a decarboxylation/Claisen condensation between malonyl-ACP (the donor of a two-carbon unit) and a growing fatty acyl CoA molecule. Notice that, again, the electrophilic acyl group is first transferred to an active site cysteine, which then serves as the leaving group in the carbon-carbon bond forming process.
Chain elongating (Claisen condensation) reaction in fatty acid biosynthesis
(step 1 in fatty acid synthesis cycle)
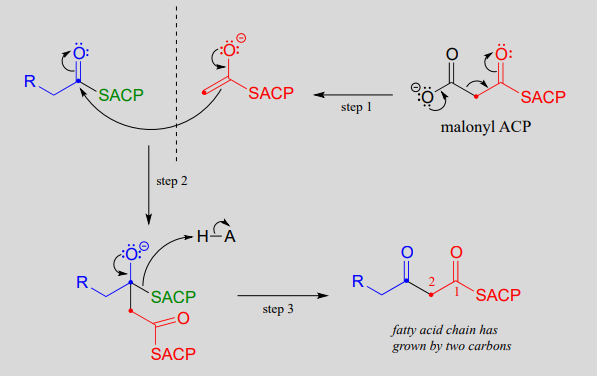
Mechanism:
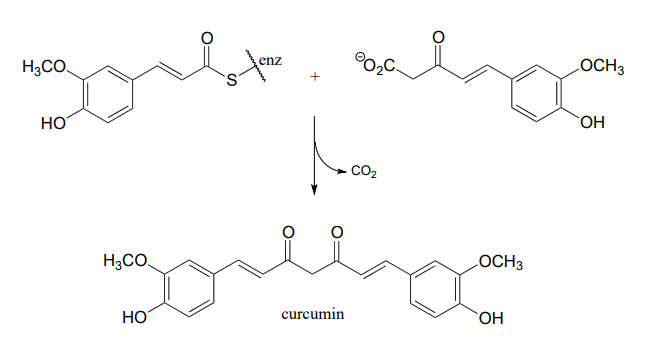
Curcumin is the compound that is primarily responsible for the distinctive yellow color of turmeric, a spice used widely in Indian cuisine. The figure below shows the final step in the biosynthesis of curcumin. Draw a mechanism for this step.
Retro-Claisen cleavage
Just like the aldol mechanism, Claisen condensation reactions often proceed in the 'retro', bond-breaking direction in metabolic pathways.
A typical Retro-Claisen cleavage reaction
(thiol nucleophile)
Mechanism:
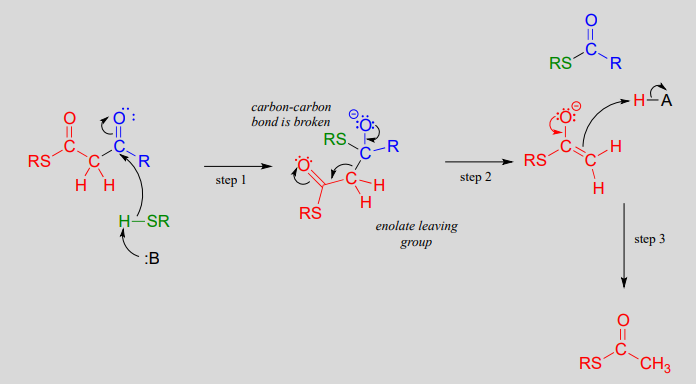
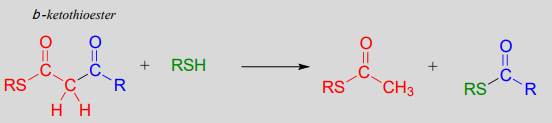
In a typical retro-Claisen reaction, a thiol (or other nucleophile such as water) attacks the carbonyl group of a \(\beta\)-thioester substrate (step 1), and then the resulting tetrahedral intermediate collapses to expel an enolate leaving group (step 2) - this is the key carbon-carbon bond-breaking step. The leaving enolate reprotonates (step 3) to bring us back to where we started, with two separate thioesters. You should look back at the general mechanism for a forward Claisen condensation and convince yourself that the retro-Claisen mechanism illustrated aboveis a step-by-step reverse process.
Is a decarboxylation/Claisen condensation step also likely to be metabolically relevant in the 'retro' direction? Explain.
When your body 'burns' fat to get energy, it is a retro-Claisen cleavage reaction (EC 2.3.1.16) that is responsible for breaking the carbon-carbon bonds in step IV of the fatty acid degradative pathway. A cysteine thiol on the enzyme serves as the incoming nucleophile (step 1 in the mechanism below), driving off the enolate leaving group as the tetrahedral intermediate collapses (step 2). The enolate is then protonated to become acetyl CoA (step 3), which goes on to enter the citric acid (Krebs) cycle. Meanwhile, a transthioesterification reaction occurs (steps a and b) to free the enzyme's cysteine residue, regenerating a fatty acyl CoA molecule which is two carbons shorter than the starting substrate.
The retro-Claisen reaction (step IV) in fatty acid degradation
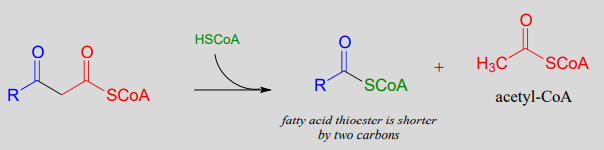
Mechanism:
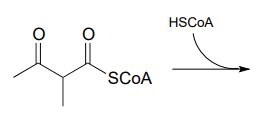
In a step in the degradation if the amino acid isoleucine, the intermediate compound 2-methyl-3-keto-butyryl \(CoA\) undergoes a retro-Claisen cleavage. Predict the products.
Many biochemical retro-Claisen steps are hydrolytic, meaning that water, rather than a thiol as in the example above, is the incoming nucleophile that cleaves the carbon-carbon bond. One example (EC 3.7.1.2) occurs in the degradation pathway for tyrosine and phenylalanine:
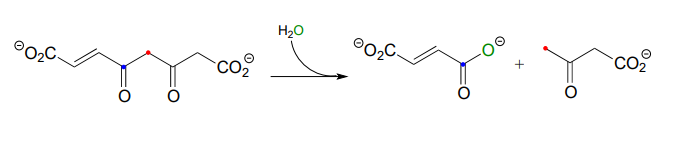
- Propose a likely mechanism for the reaction shown.
- The b-diketone substrate in the reaction above could hypothetically undergo a different retro-Claisen cleavage reaction in which the nucleophilic water attacks the other ketone group. Predict the products of this hypothetical reaction.


















