You are undoubtedly already familiar with the general idea of oxidation and reduction: you learned in general chemistry that when a compound or element is oxidized it loses electrons, and when it is reduced it gains electrons. You also know that oxidation and reduction reactions occur in tandem: if one species is oxidized, another must be reduced at the same time - thus the term 'redox reaction'.
Most of the redox reactions you have seen previously in general chemistry probably involved the flow of electrons from one metal to another, such as the reaction between copper ion in solution and metallic zinc:
\[Cu^{+2}_{(aq)} + Zn_{(s)}\rightarrow Cu_{(s)}+ Zn^{+2}_{(aq)}\]
Reading the reaction above from left to right, which chemical species is being oxidized? Which is being reduced?
When we talk about the oxidation and reduction of organic compounds, what we are mainly concerned with is the number of carbon-heteroatom bonds in the compound compared to the number of carbon-hydrogen bonds. (Remember that the term 'heteroatom' in organic chemistry generally refers to oxygen, nitrogen, sulfur, or a halogen).
- Oxidation of an organic compound results an increase in the number of carbon-heteroatom bonds, and/or a decrease in the number of carbon-hydrogen bonds.
- Reduction of an organic compound results in a decrease in the number of carbon-heteroatom bonds, and/or an increase in the number of carbon-hydrogen bonds.
Below are a number of common functional group transformations that are classified as redox.
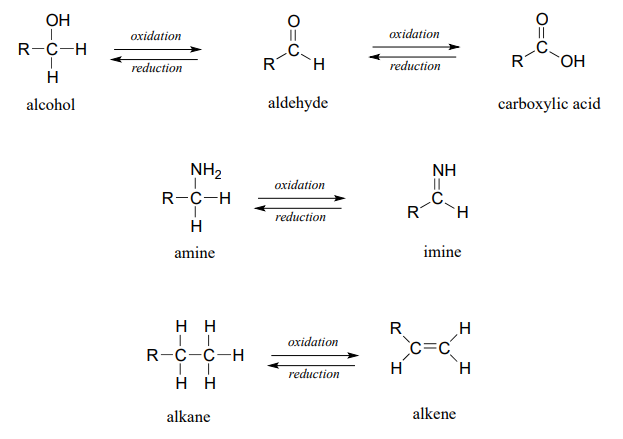
Heteroatoms such as oxygen and nitrogen are more electronegative than carbon, so when a carbon atom gains a bond to a heteroatom, it loses electron density and is thus being oxidized. Conversely, hydrogen is less electronegative than carbon, so when a carbon gains a bond to a hydrogen, it is gaining electron density, and thus being reduced.
The hydration of an alkene to an alcohol is not classified as a redox reaction. Explain.
For the most part, when talking about redox reactions in organic chemistry we are dealing with a small set of very recognizable functional group transformations. The concept of oxidation state can be useful in this context. When a compound has lots of carbon-hydrogen bonds, it is said to be in a lower oxidation state, or a more reduced state. Conversely, if it contains a lot of carbon-heteroatom bonds, it is said to be in a higher oxidation state.
We'll start with a series of single carbon compounds as an example. Methane, in which the carbon has four bonds to hydrogen, is the most reduced member of the group. The compounds become increasingly oxidized as we move from left to right, with each step gaining a bond to oxygen and losing a bond to hydrogen. Carbon dioxide, in which all four bonds on the carbon are to oxygen, is in the highest oxidation state.
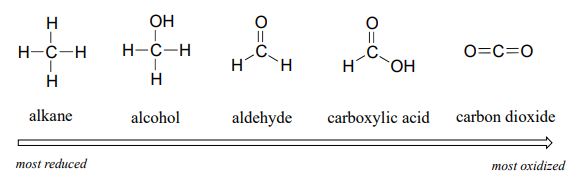
More generally, we can rank the oxidation state of common functional groups:
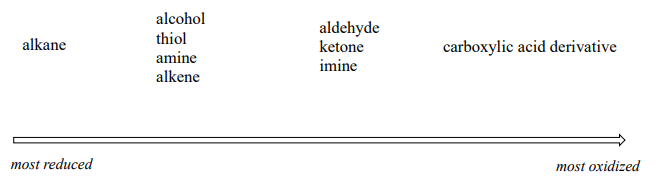
The alkane oxidation state is the most reduced. Alcohols, thiols, amines, and alkenes are all at the same oxidation state: therefore, a reaction converting one of these groups to another - an alcohol to alkene conversion, for example - is not a redox reaction. Aldehydes, however, are at a higher oxidation state than alcohols, so an alcohol to aldehyde conversion is an oxidation. Likewise, an imine to amine conversion is a reduction, but an imine to ketone conversion is not a redox reaction.
It is important to keep in mind that oxidation and reduction always occurs in tandem: when one compound is oxidized, another compound must be reduced. Often, organic chemists will use the terms oxidizing agent and reducing agent to refer to species that are commonly used, by human chemists or by nature, to achieve the oxidation or reduction of a variety of compounds. For example, chromium trioxide (\(CrO_3\)) is a laboratory oxidizing agent used by organic chemists to oxidize a secondary alcohol to a ketone, in the process being reduced to \(H_2CrO_3\). Sodium borohydride (\(NaBH_4\)) is a laboratory reducing agent used to reduce ketones (or aldehydes) to alcohols, in the process being oxidized to \(NaBH_3OH\).

There is a wide selection of oxidizing and reducing agents available for use in the organic chemistry laboratory, each with its own particular properties and uses. For example, while sodium borohydride is very useful for reducing aldehyde and ketone groups to alcohols, it will not reduce esters and other carboxylic acid derivatives. If you take a course in synthetic organic chemistry, you will learn about the use of many of these agents.
In this book, of course, we are concerned primarily with the organic chemistry that occurs within a living cell. A large part of this chapter will be spent looking at the action of two very important classes of coenzymes - the nicotinamides and the flavins - that serve as biochemical oxidizing and reducing agents. We also consider the oxidation and reduction of sulfur atoms in thiol groups, especially the thiol group on the side chain of cysteine residues in proteins.
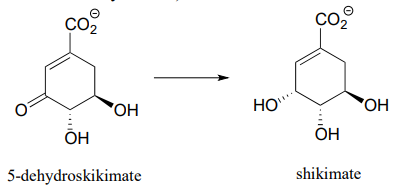
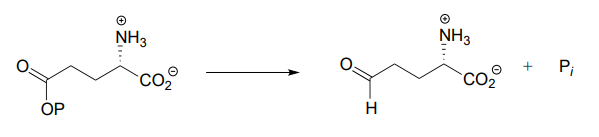
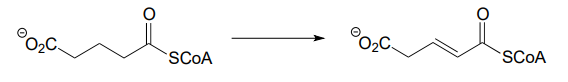
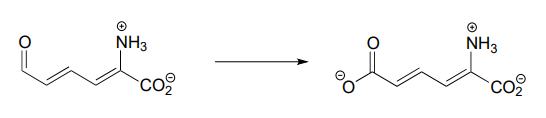
Each of the biochemical transformations shown below is a step in amino acid metabolism. For each, state whether the substrate is being oxidized, reduced, or neither oxidized nor reduced.
- (from aromatic amino acid biosynthesis)
- (from the biosynthesis of arginine and proline)
- (from the catabolism of lysine)
- (from the catabolism of tryptophan)
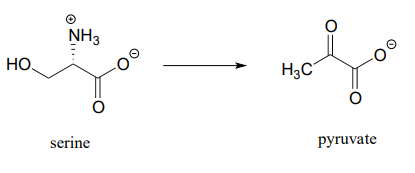
- (from the catabolism of serine)