The fact that one of the atoms adjacent to the carbonyl carbon in carboxylic acid derivatives is an electronegative heteroatom – rather than a carbon like in ketones or a hydrogen like in aldehydes - is critical to understanding the reactivity of carboxylic acid derivatives. The most significant difference between a ketone/aldehyde and a carboxylic acid derivative is that the latter has a potential leaving group - what we are calling the 'acyl X group' - bonded to the carbonyl carbon.
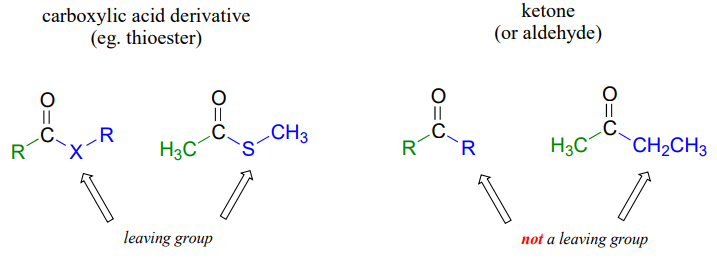
As a result, carboxylic acid derivatives undergo nucleophilic acyl substitution reactions, rather than nucleophilic additions like ketones and aldehydes.
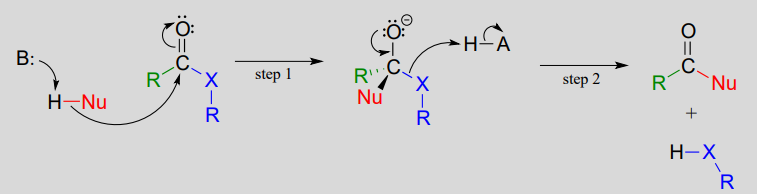
A nucleophilic acyl substitution reaction starts with nucleophilic attack at the carbonyl, leading to a tetrahedral intermediate (step 1 below). In step 2, the tetrahedral intermediate collapses and the acyl X group is expelled, usually accepting a proton from an enzymatic acid in the process.
Mechanism for a nucleophilic acyl substitution reaction:
Notice that in the product, the nucleophile becomes the new acyl X group. This is why this reaction type is called a nucleophilic acyl substitution: one acyl X group is substituted for another. For example, in the reaction below, one alcohol 'X group' (methanol), substitutes for by another alcohol 'X group' (3-methyl-1-butanol) as one ester is converted to another.
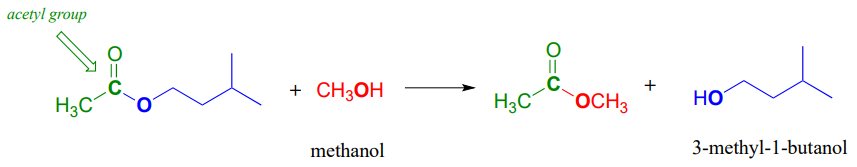
Another way of looking at this reaction is to picture the acyl group being transferred from one acyl X group to another: in the example above, the acetyl group (in green) is transferred from 3-methyl-1-butanol (blue) to methanol (red). For this reason, nucleophilic acyl substitutions are also commonly referred to as acyl transfer reactions.
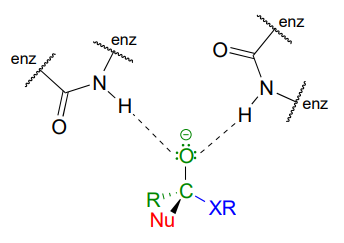
Enzymes catalyzing nucleophilic acyl substitution reactions have evolved ways to stabilize the negatively charged, tetrahedral intermediate, thus lowering the activation energy of the first, rate-determining step (nucleophilic attack). The late transition state of the first step resembles the tetrahedral intermediate that results: recall from chapter 6 that the Hammond postulate tells us that anything that stabilizes the tetrahedral intermediate will also stabilize the transition state. In many cases, for example, enzymatic amino acid residues are positioned in the active site so as to provide stabilizing hydrogen bond donating interactions with the negatively-charged oxygen. This arrangement is sometimes referred to in the biochemistry literature as an oxanion hole. The figure below shows a tetrahedral intermediate stabilized by hydrogen bond donation from two main chain (amide) nitrogen atoms.