4.7: Multiple Bonds in VB Theory
- Page ID
- 36141
Learning Objectives
- To explain multiple bonds in terms of hybridized and unhybridized atomic orbitals according to Valence Bond Theory.
So far in our bonding descriptions according to Valence Bond Theory, we have not dealt with polyatomic systems with multiple bonds. To do so, we can use an approach in which we describe \(\sigma\) bonding using localized electron-pair bonds formed by hybrid atomic orbitals, and \(\pi\) bonding using unhybridized np atomic orbitals.
Non-singular Bonding
We begin our discussion by considering the bonding in ethylene (C2H4). Experimentally, we know that the H–C–H and H–C–C angles in ethylene are approximately 120°. This angle suggests that the carbon atoms are sp2 hybridized, which means that a singly occupied sp2 orbital on one carbon overlaps with a singly occupied s orbital on each H and a singly occupied sp2 lobe on the other C. Thus each carbon forms a set of three \(\sigma\) bonds: two C–H (sp2 + s) and one C–C (sp2 + sp2) (part (a) in Figure 4.7.1). The sp2 hybridization can be represented as follows:
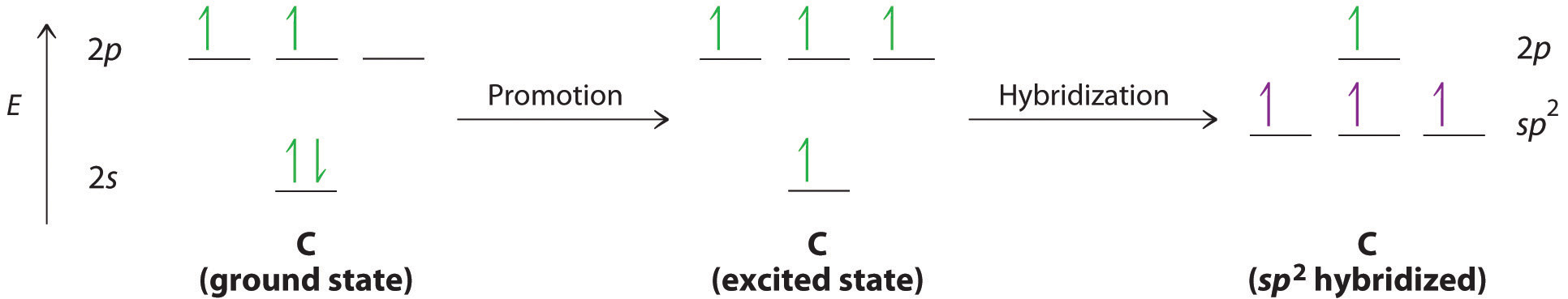
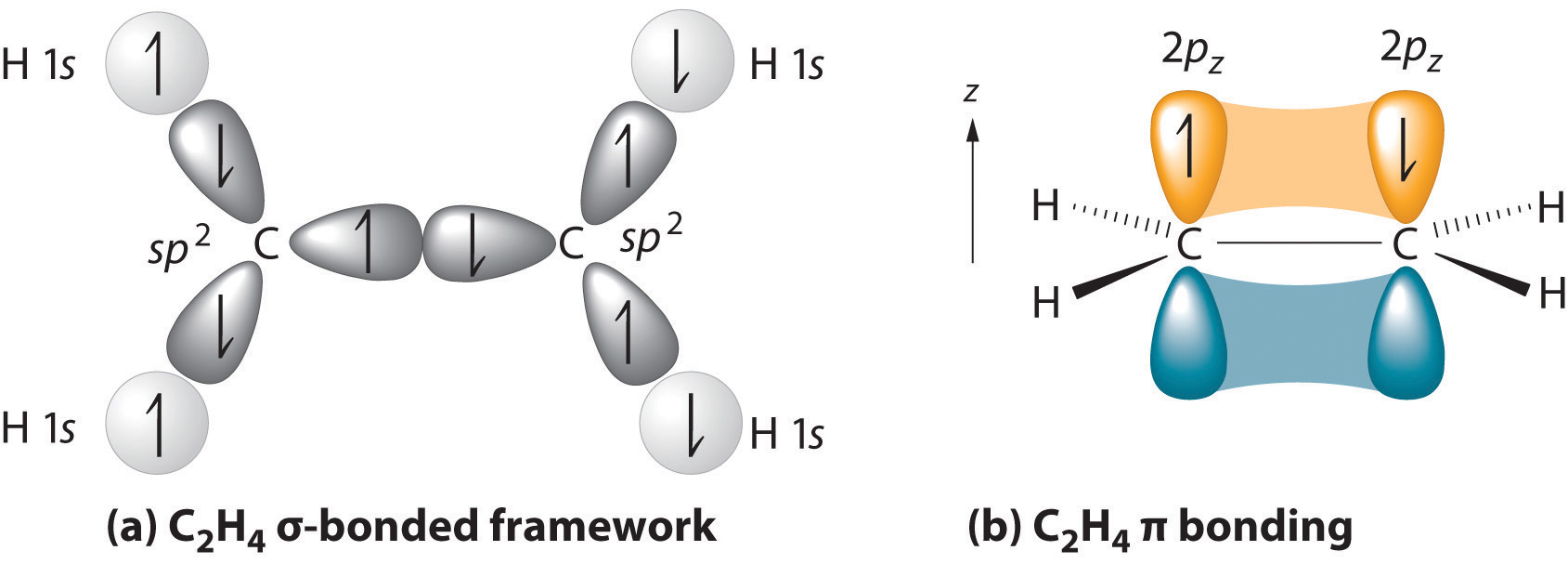
After hybridization, each carbon still has one unhybridized 2pz orbital that is perpendicular to the hybridized lobes and contains a single electron (part (b) in Figure 4.7.1). The two singly occupied 2pz orbitals can overlap to form a \(\pi\) bond. This is only possible when the two orbitals are arranged parallel. Rather than electron density along the internuclear axis, the \(\pi\) bond has a nodal plane along the internuclear axis with the electron density above and below the axis. Because each 2pz orbital has a single electron, there are two electrons in the \(\pi\) bond. Consequently, the C–C bond in ethylene consists of a \(\sigma\) bond and a \(\pi\) bond, which together give a C=C double bond. Thus, the bond order between the two carbon atoms is 2.
Our model is supported by the facts that the measured carbon–carbon bond is shorter than that in ethane (133.9 pm versus 153.5 pm) and the bond is stronger (728 kJ/mol versus 376 kJ/mol in ethane). The two CH2 fragments are coplanar, which maximizes the overlap of the two singly occupied 2pz orbitals.
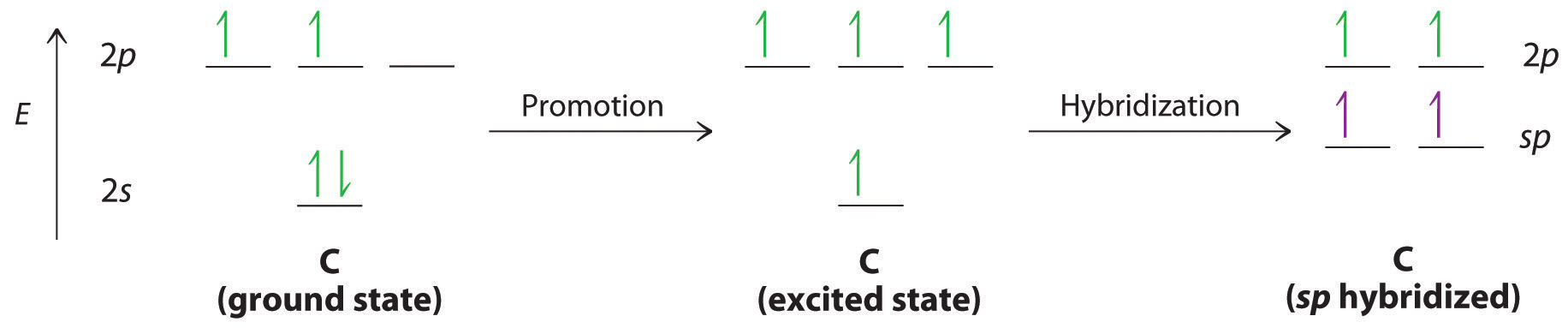
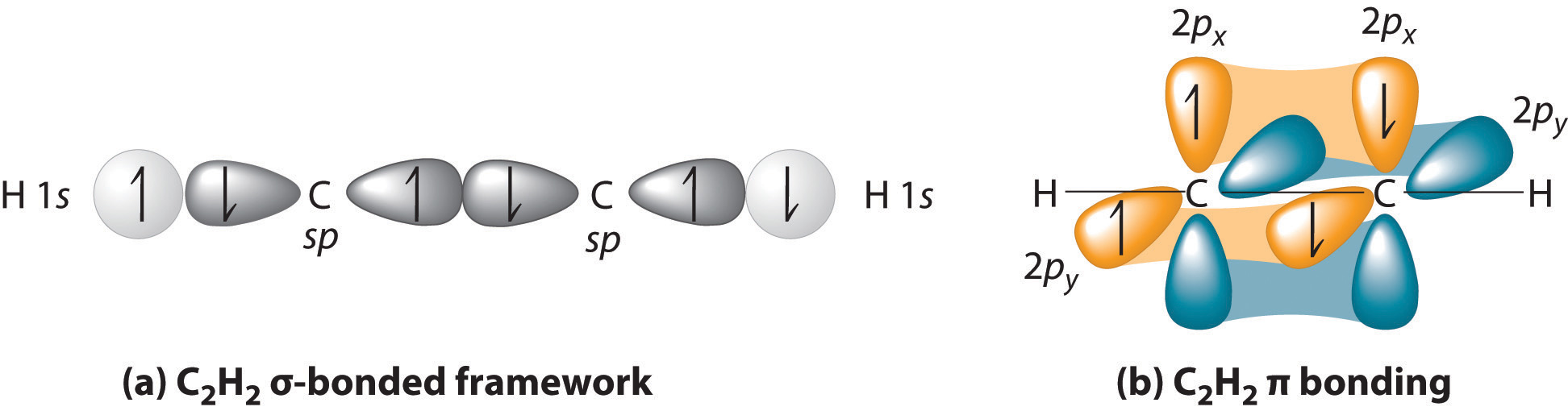
Now, consider triple bonds, as in acetylene (C2H2). Here the four atoms of acetylene are collinear, which suggests that each carbon is sp hybridized. If one sp lobe on each carbon atom is used to form a C–C \(\sigma\) bond and one is used to form the C–H \(\sigma\) bond, then each carbon will still have two unhybridized 2p orbitals (a 2px,y pair), each with one electron (part (a) in Figure 4.7.3).
Note
In complex molecules, hybrid orbitals and valence bond theory can be used to describe \(\sigma\) bonding, and unhybridized \(\pi\) orbitals can be used to describe \(\pi\) bonding.
Example 4.7.1
Describe the bonding in HCN using a combination of hybrid atomic orbitals and unhybridized atomic orbitals. The HCN molecule is linear.
Given: chemical compound and molecular geometry
Asked for: bonding description using hybrid atomic orbitals and unhybridized orbitals
Strategy:
- From the geometry given, predict the hybridization in HCN. Use the hybrid orbitals to form the \(\sigma\)-bonded framework of the molecule and determine the number of valence electrons that are used for \(\sigma\) bonding.
- Determine the number of remaining valence electrons. Use any remaining unhybridized p orbitals to form \(\pi\) bonds.
- Describe the bonding in HCN.
Solution:
A Because HCN is a linear molecule, it is likely that the bonding can be described in terms of sp hybridization at carbon. Because the nitrogen atom can also be described as sp hybridized, we can use one sp hybrid on each atom to form a C–N \(\sigma\) bond. This leaves one sp hybrid on each atom to either bond to hydrogen (C) or hold a lone pair of electrons (N). Of 10 valence electrons (5 from N, 4 from C, and 1 from H), 4 are used for \(\sigma\) bonding:
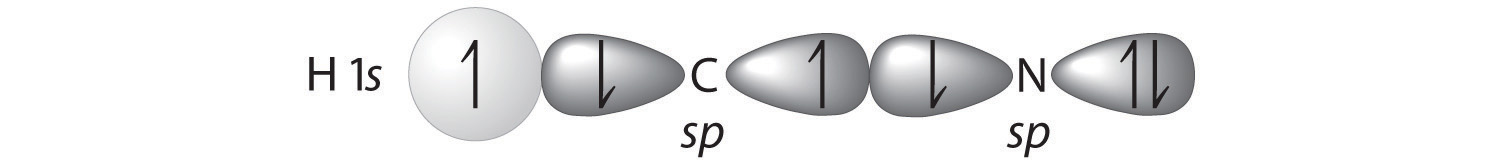
B We are now left with 2 electrons on N (5 valence electrons minus 1 bonding electron minus 2 electrons in the lone pair) and 2 electrons on C (4 valence electrons minus 2 bonding electrons). We have two unhybridized 2p atomic orbitals left on carbon and two on nitrogen, each occupied by a single electron. These four 2p atomic orbitals can be arranged in two pairs of parallel orbitals, yielding two \(\pi\) bonds.
C The overall result is a triple bond (1 \(\sigma\) and 2 \(\pi\)) between C and N.
Exercise
Describe the bonding in formaldehyde (H2C=O), a trigonal planar molecule, using a combination of hybrid atomic orbitals and unhybridized orbitals.
- Answer
-
- \(\sigma\)-bonding framework: Carbon and oxygen are sp2 hybridized. Two sp2 hybrid orbitals on oxygen have lone pairs, two sp2 hybrid orbitals on carbon form C–H bonds, and one sp2 hybrid orbital on C and O forms a C–O \(\sigma\) bond.
- \(\pi\) bonding: Unhybridized, singly occupied 2p atomic orbitals on carbon and oxygen interact to form a single \(\pi\) bond.
Once we have explored Molecular Orbital Theory we will revisit this description of multiple bonds to better understand the interactions of np orbitals to form \(\pi\) bonding type interactions.
Labeling Bonds in Valence Bond Theory
In VB Theory, we can describe each of the bonds within a molecule in terms of both the bond symmetry (\(\sigma\) or \(\pi\)) and the orbitals used to form the bond. Consider the bonding in ethylene (C2H4, Figure 4.7.1). The two carbon atoms are sp2 hybridized, while the four hydrogen atoms' 1s orbitals remain unhybridized with a single electron in each 1s atomic orbital. The four \(\sigma\) bonds in ethylene can therefore be described as follows:
\(\sigma\) : C(sp2) - H(1s)
The double bond between the two carbon atoms is made up of one \(\sigma\) bond and one \(\pi\) bond. The \(\sigma\) bond results from overlapping sp2 orbitals on the neighbouring carbon atoms. The single \(\pi\) bond is formed by overlap between parallel unhybridized 2p orbitals on the neighbouring carbon atoms. Thus, the double bond can be described as follows:
\(\sigma\) : C(sp2) - C(sp2)
\(\pi\) : C(2p) - C(2p)
Exercise
Label the bonds in formaldehyde (H2C=O), in terms of the bond symmetry and the overlapping orbitals.
- Answer
-
- The two carbon-hydrogen bonds are:
\(\sigma\) : C(sp2) - H(1s) - The carbon-oxygen double bond is:
\(\sigma\) : C(sp2) - O(sp2)
\(\pi\) : C(2p) - O(2p)
- The two carbon-hydrogen bonds are:
Summary
- Polyatomic systems with multiple bonds can be described using hybrid atomic orbitals for \(\sigma\) bonding and unhybridized np orbitals to describe \(\pi\) bonding.
To describe the bonding in more complex molecules with multiple bonds, we can use an approach that uses hybrid atomic orbitals to describe the \(\sigma\) bonding and unhybridized np orbitals to describe the \(\pi\) bonding. In this approach, parallel unhybridized np orbitals on atoms bonded to one another are allowed to interact to produce bonding.

