3.10: 3.33 Acetals and ketals
- Page ID
- 38633
When a hemiacetal (or hemiketal) is subjected to nucleophilic attack by a second alcohol molecule, the result is called an acetal (or ketal).
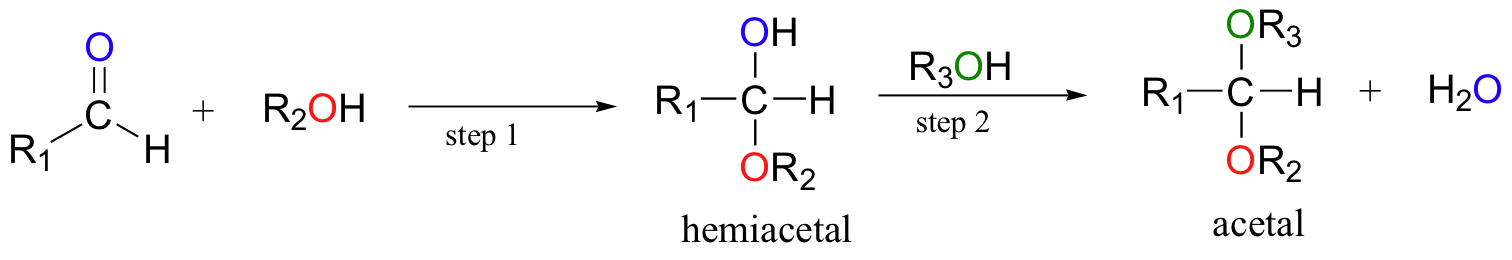
While the formation of a hemiacetal from an aldehyde and an alcohol (step 1 above) is a nucleophilic addition, the formation of an acetal from a hemiacetal (step 2 above) is a nucleophilic substitution reaction, with the original carbonyl oxygen leaving as a water molecule.
Recall from section 9.2 the structure of the glycosidic bond between two glucose molecules in a cellulose chain:
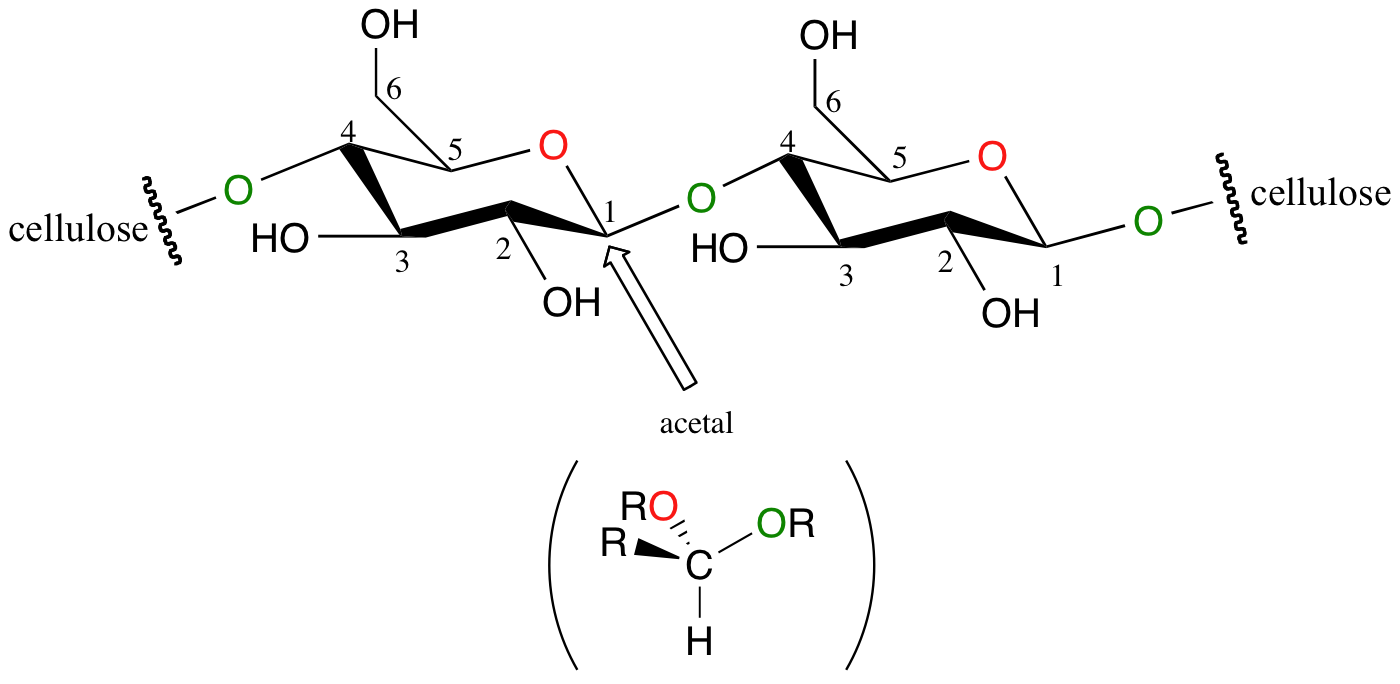
If you look carefully at C1 of each glucose unit, you should recognize that this glycosidic bond is, in fact, an acetal.
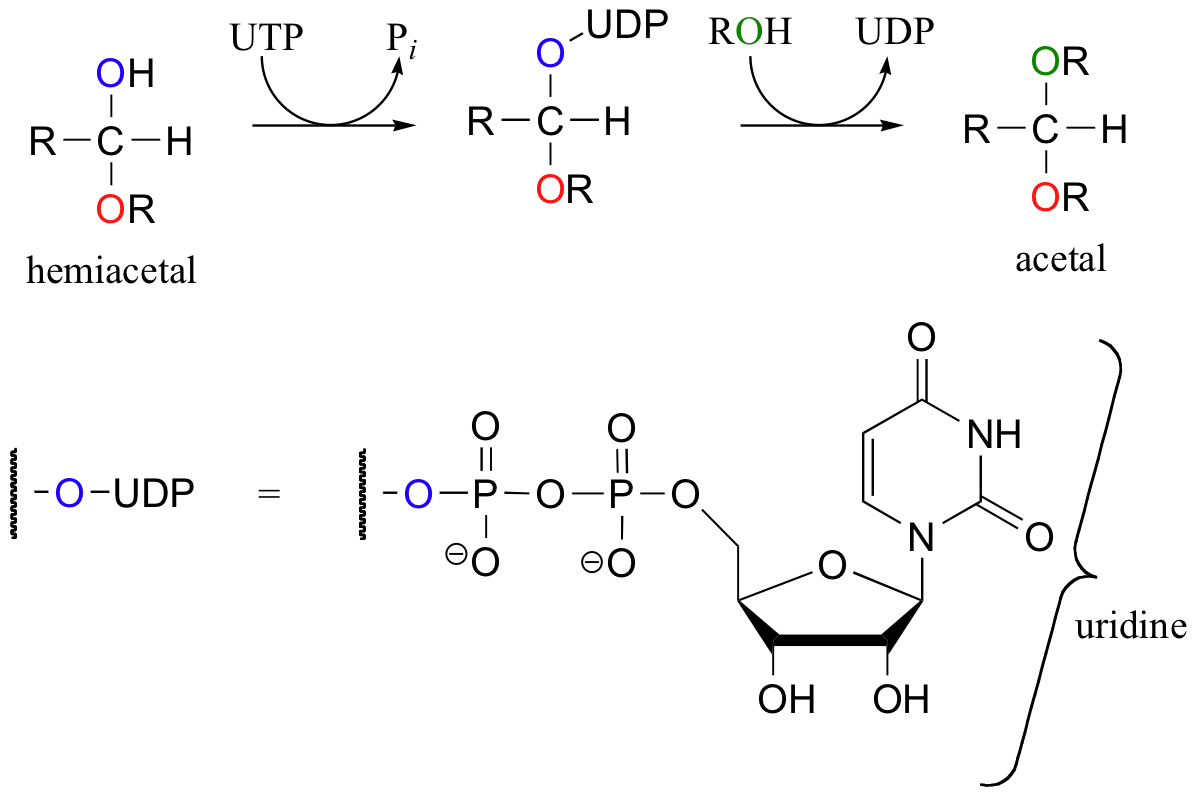
The formation of the glycosidic bond in cellulose and other carbohydrates - a hemiacetal to acetal conversion - is catalyzed by a class of enzyme called glycosyltransferases. In a glycosyltransferase reaction, the carbonyl oxygen does not leave as a water molecule, but rather as part of a uridine nucleotide diphosphate group. This represents another way to convert water into a better leaving group.
Exercise 11.1
In the first reaction of the scheme shown above, uridine triphosphate (UTP) serves as a phosphoryl group donor. Which phosphate group is the electrophile in this step, the alpha, beta, or gamma?
Let's take a look at the formation of a new glucose-glucose acetal bond in cellulose synthesis as an example. The anomeric carbon of a glucopyranose -UDP derivative is attacked from above by an alcohol, specifically the hydroxyl on C4 of the terminal glucose on the growing cellulose chain. The UDP leaving group is displaced, and inversion of stereochemistry results at the anomeric carbon.
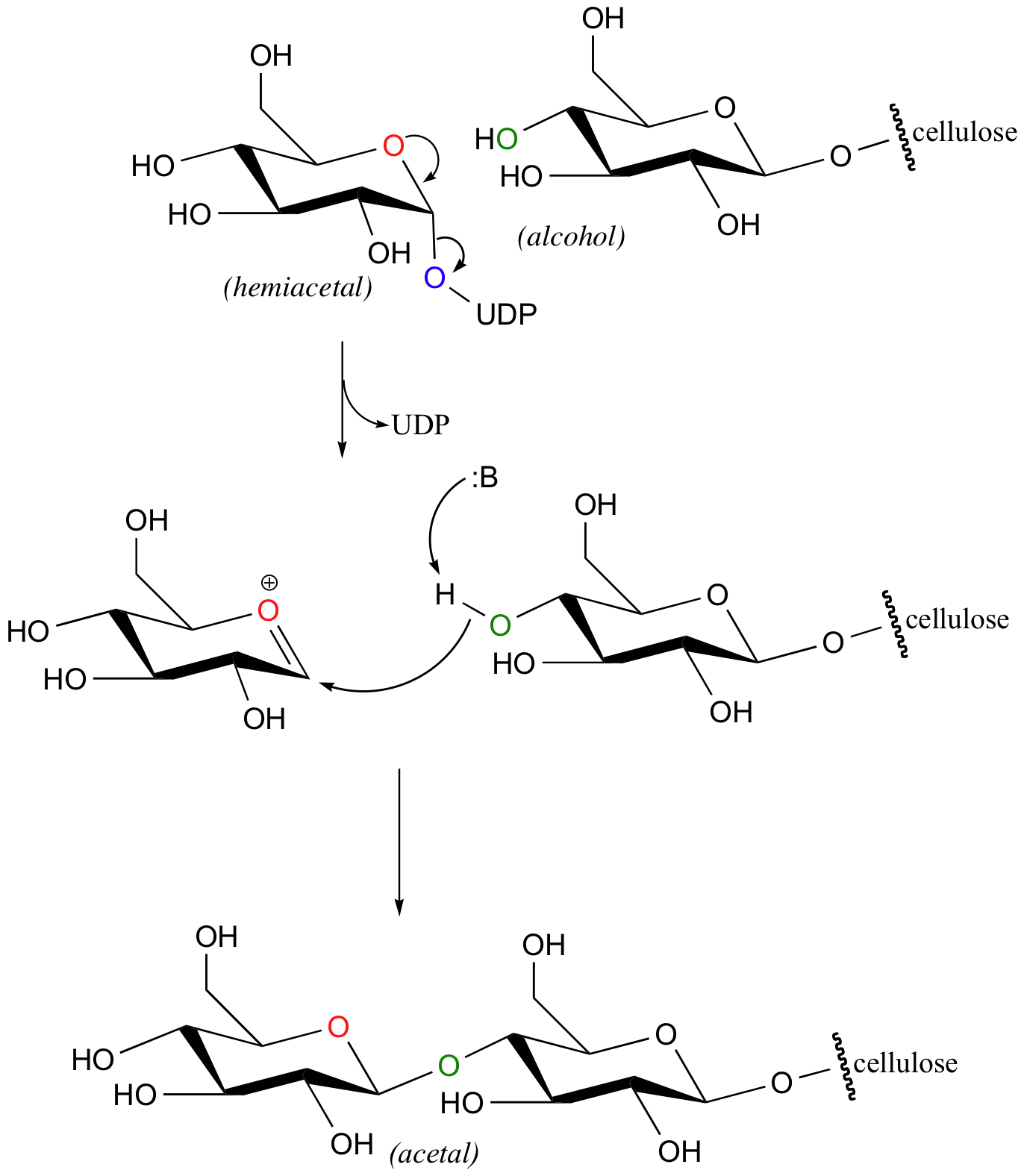
You may recognize the similarity of this reaction to the glycosidase reactions we studied earlier (section 9.2). Here, though, the attacking nucleophile is an alcohol group rather than a water molecule.
Glycosyltransferase reactions can also take place either with inversion of configuration at the anomeric carbon (such as in the reaction pictured above) or with retention of configuration. However, it is not yet clear whether 'retaining glycosyltransferase' reactions proceed through a double-displacement mechanism like the retaining glycosidases we have already studied, or though some alternate mechanism. See Nature Structural Biology 2001, 8, 98 for an interesting discussion of this problem.

