24.1: Ideal Solutions - Raoult's Law
- Page ID
- 238273
When two substances whose molecules are very similar form a liquid solution, the vapor pressure of the mixture is very simply related to the vapor pressures of the pure substances. Suppose, for example, we mix 1 mol benzene with 1 mol toluene as shown in the figure below.
The mole fraction of benzene, \(X_b\), and the mole fraction of toluene, \(X_t\), are both equal to 0.5. At 79.6°C the measured vapor pressure of this mixture is 516 mmHg, slightly less than 517 mmHg, the average of the vapor pressures of pure benzene (744 mmHg) and of pure toluene (290 mmHg) at the same temperature.
It is easy to explain this behavior if we assume that because benzene and toluene molecules are so nearly alike, they behave the same way in solution as they do in the pure liquids. Since there are only half as many benzene molecules in the mixture as in pure benzene, the rate at which benzene molecules escape from the surface of the solution will be half the rate at which they would escape from the pure liquid. In consequence the partial vapor pressure of benzene above the mixture will be one-half the vapor pressure of pure benzene. By a similar argument the partial vapor pressure of the toluene above the solution is also one-half that of pure toluene. Accordingly, we can write
\[P_b =\frac{1}{2} P_b^*\]
and
\[P_t=\frac{1}{2} P_t^*\]
where \(P_b\) and \(P_t\) are the partial pressures of benzene and toluene vapors, respectively, and \(P_b^*\) and \(P_y^*\) are the vapor pressures of the pure liquids. The total vapor pressure of the solution is
\[P=P_b+P_t=\frac{1}{2}P_b^{*}+\frac{1}{2}P_t^{*}=\frac{P_b^{*}+P_t^{*}}2\]
The vapor pressure of the mixture is equal to the mean of the vapor pressures of the two pure liquids.
We can generalize the above argument to apply to a liquid solution of any composition involving any two substances \(A\) and \(B\) whose molecules are very similar. The partial vapor pressure of \(A\) above the liquid mixture, \(P_A\), will then be the vapor pressure of pure \(A\), \(P_A^*\), multiplied by the fraction of the molecules in the liquid which are of type \(A\), that is, the mole fraction of \(A\), \(X_A\). In equation form
\[P_A=X_AP_A^* \label{3}\]
Similarly for component \(B\)
\[P_B=X_BP_B^* \label{4}\]
Adding these two partial pressures, we obtain the total vapor pressure
\[P=P_A + P_B = X_AP_A^* + X_BP_B^* \label{5}\]
Liquid solutions which conform to Eqs. \(\ref{3}\) and \(\ref{5}\) are said to obey Raoult’s law and to be ideal mixtures or ideal solutions.
In addition to its use in predicting the vapor pressure of a solution, Raoult’s law may be applied to the solubility of a gas in a liquid. Dividing both sides of Equation \(\ref{3}\) by \(P_A^*\) gives
\[X_A=\frac{1}{P_A^{*}}\times P_A=k_A\times P_A\label{6}\]
Since the vapor pressure of any substance has a specific value at a given temperature, Equation \(\ref{6}\) tells us that the mole fraction \(X_A\) of a gaseous solute is proportional to the partial pressure \(P_A\) of that gas above the solution.
For an ideal solution the proportionality constant \(k_A\) is the reciprocal of the vapor pressure of the pure solute at the temperature in question. Since vapor pressure increases as temperature increases, \(k_A\), which is \(1/P_A^*\), must decrease. Thus we expect the solubility of a gas in a liquid to increase as the partial pressure of gas above the solution increases, but to decrease as temperature increases. Equation \(\ref{6}\) is known as Henry’s law. It also applies to gaseous solutes which do not form ideal solutions, but in such cases the Henry’s-law constant \(k_A\) does not equal the reciprocal of the vapor pressure.
The video below shows the effect of varied pressure on the amount of CO2 dissolved in soda. The amount of dissolved CO2 is monitored by a pH indicator. The more dissolved CO2, the lower the pH (the more red the solution). Watch the video to find out how the solubility of CO2 is related to the pressure, paying particular attention to the color of the solution.
In actual fact very few liquid mixtures obey Raoult’s law exactly. Even for molecules as similar as benzene and toluene, we noted a deviation of 517 mmHg – 516 mmHg, or 1 mmHg at 79.6°C. Much larger deviations occur if the molecules are not very similar. These deviations are of two kinds. As can be seen from Figure \(\PageIndex{2}\) , a plot of the vapor pressure against the mole fraction of one component yields a straight line for an ideal solution. For non-ideal mixtures the actual vapor pressure can be larger than the ideal value (positive deviation from Raoult’s law) or smaller (negative deviation). Negative deviations correspond to cases where attractions between unlike molecules are greater than those between like molecules.
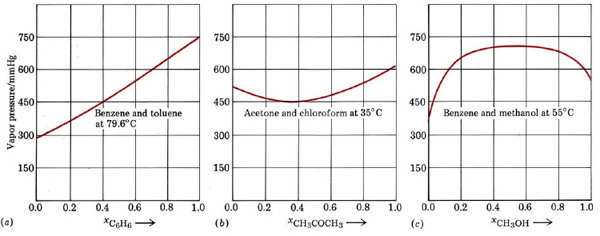
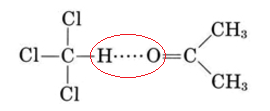
Because of this extra intermolecular attraction, molecules have more difficulty escaping the solution and the vapor pressure is lower. The opposite is true of a mixture of benzene and methanol. When C6H6 molecules are randomly distributed among CH3OH molecules, the latter cannot hydrogen bond effectively. Molecules can escape more readily from the solution, and the vapor pressure is higher than Raoult’s law would predict.
Contributors
Ed Vitz (Kutztown University), John W. Moore (UW-Madison), Justin Shorb (Hope College), Xavier Prat-Resina (University of Minnesota Rochester), Tim Wendorff, and Adam Hahn.

