6.4: Oxidation–Reduction Reactions
- Page ID
- 393895
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Define oxidation, reduction, oxidizing agents, and reducing agents.
- Recognize a reaction as an oxidation-reduction reaction.
- Identify the elements reduced or oxidized in a redox reaction.
- Identify oxidizing and reducing agents.
Oxidation-Reduction - Transfer of Electrons
In the course of a chemical reaction between a metal and a nonmetal, electrons are transferred from the metal atoms to the nonmetal atoms. For example, when zinc metal is mixed with sulfur and heated, the compound zinc sulfide is produced. Two valence electrons from each zinc atom are transferred to each sulfur atom.

Since the zinc is losing electrons in the reaction, it is being oxidized. The sulfur is gaining electrons and is thus being reduced. An oxidation-reduction reaction is a reaction that involves the full or partial transfer of electrons from one reactant to another. Oxidation is the full or partial loss of electrons or the gain of oxygen. Reduction is the full or partial gain of electrons or the loss of oxygen. A redox reaction is another term for an oxidation-reduction reaction.
An element is oxidized when it loses electrons.
An element is reduced when it gains electrons.
Each of these processes can be shown in a separate equation called a half-reaction. A half-reaction is an equation that shows either the oxidation or the reduction reaction that occurs during a redox reaction.
\[ \underbrace{\ce{Zn→Zn^{2+}+2e^{−}}}_{\text{Oxidation}} \label{7.9.1} \]
\[ \underbrace{\ce{S+ 2 e^{−} → S^{2−}}}_ {\text{Reduction}} \label{7.9.2} \]
It is important to remember that the two half-reactions occur simultaneously. The resulting ions that are formed are then attracted to one another in an ionic bond.
Another example of an oxidation-reduction reaction involving electron transfer is the well-known combination of metallic sodium and chlorine gas to form sodium chloride:
\[\ce{2Na+Cl_2→2NaCl} \label{7.9.3} \]
The half-reactions are as follows:
\[ \underbrace{\ce{2Na→2Na^{+} + 2e^{−}}}_{\text{Oxidation}} \label{7.941} \]
\[ \underbrace{\ce{Cl_2 +2e^{−} → 2Cl^{−}}}_ {\text{Reduction}} \label{7.9.5} \]
Identify what is being oxidized and reduced in the following redox reaction.
\[\ce{2Na + Br2 → 2NaBr} \nonumber \]
Solution
Both reactants are the elemental forms of their atoms, so the Na and Br atoms have a charge of 0. In the ionic product, the Na+ ions have a charge of +1, while the Br− ions have a cahrge of −1.
\[2\underset{0}{Na}+\underset{0}{Br_{2}}\rightarrow 2\underset{+1 -1}{NaBr} \nonumber \]
Sodium is increasing its charge from 0 to +1, so it is being oxidized; bromine is decreasing its charge from 0 to −1, so it is being reduced:
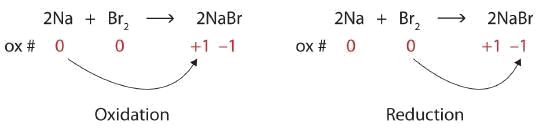
Because charges are changing, this is a redox reaction. The total number of electrons being lost by sodium (two, one lost from each Na atom) is gained by bromine (two, one gained for each Br atom).
We often talk about species that cause something to be oxidized or reduced because we may need to create a specific change in a compound. For example, a common way to remove stains in clothing is to oxidize the stain, which requires a compound that causes oxidation. A compound that causes oxidation is an oxidizing agent. Conversely, a reducing agent is a compound that causes reduction.
Oxidation-Reduction with Oxygen and Hydrogen
Oxidation and reduction can also be defined in terms of changes in composition. The original meaning of oxidation was “adding oxygen,” so when oxygen is added to a molecule, the molecule is being oxidized. The reverse is true for reduction: if a molecule loses oxygen atoms, the molecule is being reduced. For example, the acetaldehyde (\(\ce{CH3CHO}\)) molecule takes on an oxygen atom to become acetic acid (\(\ce{CH3COOH}\)).
\[\ce{2CH3CHO + O2 → 2CH_3COOH} \nonumber \]
Thus, acetaldehyde is being oxidized.
Similarly, reduction and oxidation can be defined in terms of the gain or loss of hydrogen atoms. If a molecule adds hydrogen atoms, it is being reduced. If a molecule loses hydrogen atoms, the molecule is being oxidized. For example, in the conversion of acetaldehyde into ethanol (\(\ce{CH3CH2OH}\)), hydrogen atoms are added to acetaldehyde, so the acetaldehyde is being reduced:
\[\ce{CH3CHO + H2 → CH3CH2OH} \nonumber \]
| Process | Change in oxygen (some reactions) | Change in hydrogen (some reactions) |
|---|---|---|
| Oxidation | gain | lose |
| Reduction | lose | gain |
In each conversion, indicate whether oxidation or reduction is occurring.
- N2 → NH3
- CH3CH2OHCH3 → CH3COCH3
- HCHO → HCOOH
Solution
- Hydrogen is being added to the original reactant molecule, so reduction is occurring.
- Hydrogen is being removed from the original reactant molecule, so oxidation is occurring.
- Oxygen is being added to the original reactant molecule, so oxidation is occurring.
In each conversion, indicate whether oxidation or reduction is occurring.
- CH4 → CO2 + H2O
- NO2 → N2
- CH2=CH2 → CH3CH3
- Answer a:
-
Oxygen is being added. Oxidation is occurring.
- Answer b:
-
Oxygen is being removed. Reduction is occurring.
- Answer a:
-
Hydrogen is being added. Reduction is occurring.
Key Takeaway
Chemical reactions in which electrons are transferred are called oxidation-reduction, or redox, reactions. Oxidation is the loss of electrons. Reduction is the gain of electrons. Oxidation and reduction always occur together, even though they can be written as separate chemical equations.
Contributors & Affiliations

